Compassionate Drugs: A reason for hope and smile


In India, “compassionate use” is an unceremonious term usually used by individuals in their conversation for better understanding whereas “Expanded access” is an official term designated by the United States Food and Drug Administration (US FDA).…..
By Geeta Aggarwal and Nidhi Tiwary
Imagine if you have suffered from a life-threatening disease and your physician already informed you that in attempting to treat your illness, all established treatments have been tried but all are unsuccessful. Many patients and families leave their hope for alive and to receive palliative care attempt to provide the best feasible end of life. On the other hand, just visualize if your physician informed you that they may be aware of experimental drugs used for the same purpose as yours and yet to be under clinical trials.
Moreover, this drug has been showing favorable effects in animal testing during laboratory under phase 1 and 2 trials. The patients might set their new ray of hopes on the experimental drugs with a compassionate smile to know about enhance their life span and mean of survival time. Another question is still in the patient’s mind, how would like to access experimental drugs? What are the various options? What are the eligibility criteria to get it?? Previous studies showed whenever a new therapeutic intervention is in a development phase, it has to be pass under several phases of clinical testing on humans (phase 0-4) and will take almost 10-12 years for market approval. Nowadays, there are many guidelines set up by the US FDA for seriously ill patients to access investigational drugs that have not yet gained market approval using expanded access and compassionate use programs by participating in randomized controlled trials (RCT). According to the World Health Organization (WHO), Compassionate use (CU) is a “program that is intended to provide potentially life-saving experimental treatments to patients suffering from a disease for which no satisfactory authorized therapy exists and/or who cannot enter a clinical trial. For many patients, these programs represent their last hope. Currently, many terms are used interchangeably for Compassionate drug use” such as “Compassionate use,” “Expanded access,” “Treatment use,” “Special access,” “Pre-approval assess,” “Emergencyuse” and “Access”.
In India, “compassionate use” is an unceremonious term usually used by individuals in their conversation for better understanding whereas “Expanded access” is an official term designated by the United States Food and Drug Administration (US FDA). There are certain conditions when expanded or compassionate use of the drug is authorized by USFDA: (1) Is the patient suffering from a chronic and life-threateningsituation? (2) Unavailability of drug therapy or any kind of prophylactic treatment to diagnose, monitor, and treat the diseases. (3) Registration of patient’s is not feasible in clinical trials. (4) To ensure investigational drug will not interfere with phases of clinical trials which is conducted for the marketing approval of a drug for the treatment sign [1].
Traditionally, a drug is reachable to patients, only if an adequate amount of data has been proved during clinical trials regarding its safety, adverse effects, and achievable good pharmacokinetic and pharmacodynamic profile w.r.t its efficacy. Sometimes, this approach creates trouble in the time of crisis and leads to multiple disability and fatality rate due to extensive and strenuous phases of clinical trials. The various pathway involved in compassionate use program are summarized in
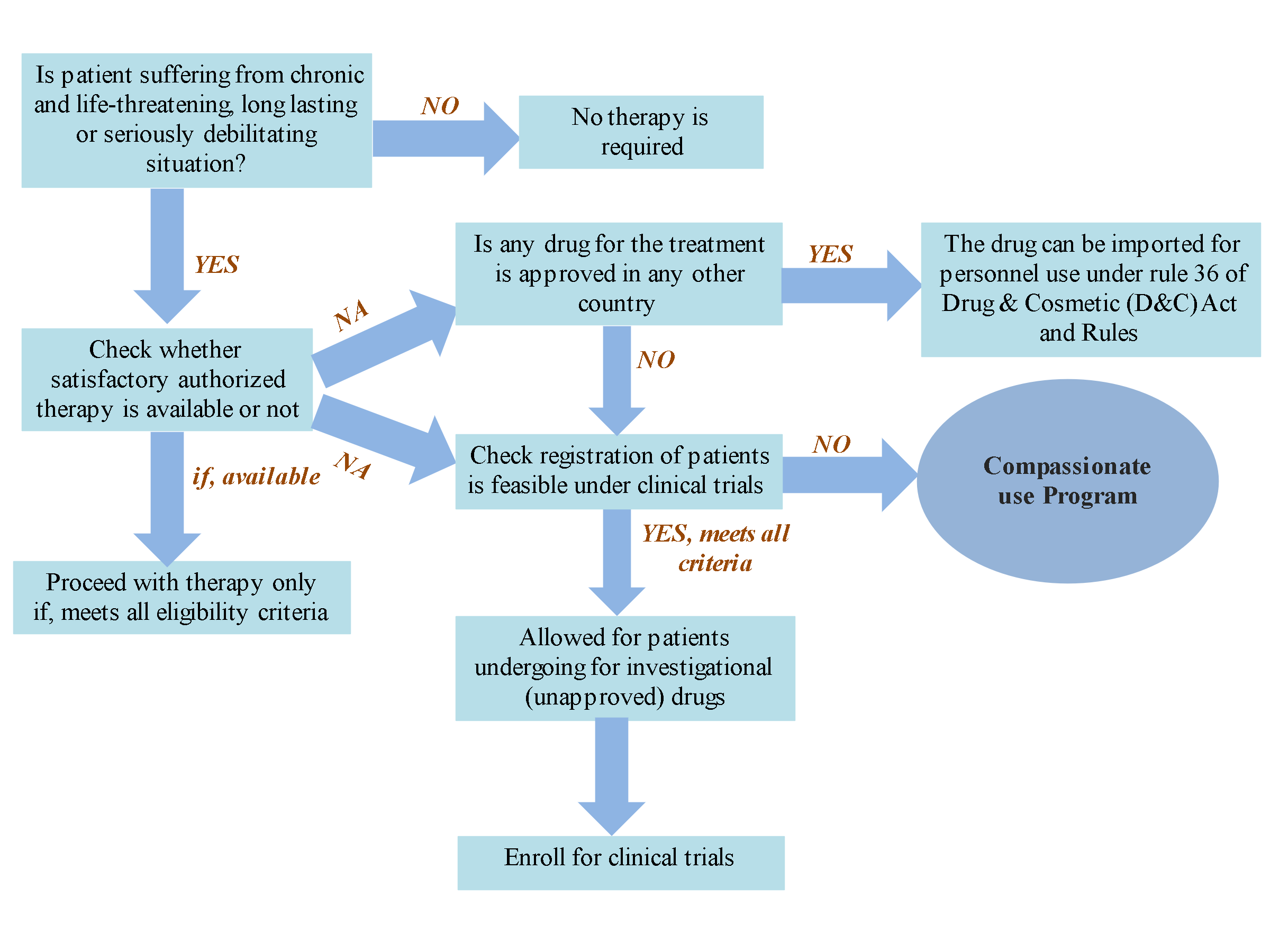 Fig.1.
Fig.1.
In 2019, during coronavirus (COVID-19) outbreak,this major problem was noticed by the Government under the Ministry of Health and Family Welfare in India and it made a draft for taking amendment in New Drugs and Clinical Trials Rules, 2019 and introduced the regulatory framework for manufacture and import of unapproved/unauthorized novel drugs for ‘emergency use’. In the pandemic era (COVID-19), a crucial step has been taken by regulatory bodies to review the investigational drugs and to provide their access as no other drug therapy was available for its prevention. It is a diversion from conventional approach to modern approach and is an enormous step for the prevention and treatment of any type of crisis/dangerous situation in upcoming years and a lifetime.
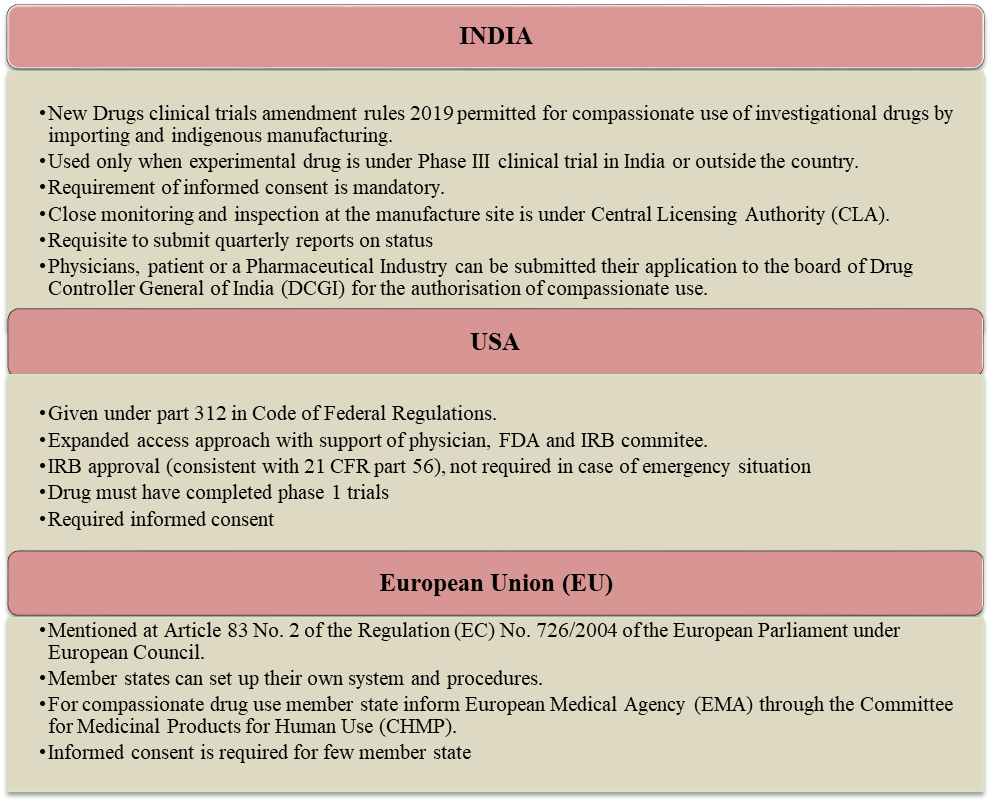
Fig.1. Flow diagram depicts compassionate use program for patients suffering from life-threatening conditions which are more likely to progress to more serious condition without early treatment. (Abbreviations: NA- Not available).
Involvement of Regulatory Bodies in Compassionate use program
There are several guidelines followed by regulatory bodies under access to unapproved/experimental drugs in different parts of the country. Compassionate use is a different kind of treatment and must require reviewing by ethics committee. Some of the countries’ key differences regarding regulations of compassionate use are summarized in Fig.2. In addition, other countries like Germany, Netherlands, Norway, and Spain already invented their own nationalized guidelines and rules for compassionate program [2].
Fig.2.Comparison between regulatory outline for compassionate use of unapproved drugs in different countries (India/USA/EU)

Compassionate Drugs in Diseases[3,4]
1. Coronavirus (COVID-19)
As you all are aware the outburst Coronavirus disease 2019 (COVID-19) is caused by coronavirus-2 (SARS-CoV-2; formerly called 2019-nCoV). It was initially reported by World Health Organization (WHO)and later it has been declared a global health emergency on January 30, 2020. Subsequently on March 11, 2020, WHO declared COVID-19 a global pandemic, its first such designation since declaring H1N1 influenza pandemic in 2009. There arevarious programs accepted by USFDA to allow clinicians and researchers to gain access to investigational/experimental therapies during the coronavirus pandemic. The compassionate access and emergency use authorization (EUA) programs allowed for use of investigational drug therapies with promising evidencein emergency cases.
 The development of novel therapeutic interventions and vaccines for treatment within a short time span was one of the biggest challenges for the pharmaceutical industry as well as for the medical expert’s team because of unexpected outcomes with COVID-19 spread. Considering immediate unmet needs, all medical faculties,scientists, researchers, and government bodiesscannedthe existing drugs based on their symptomatology and their toxicity profile forrepurposing and explore their alternate use. The repurposed drugs were used in the line of compassionate use of drugs. Potential candidates for repurposing includeda variety of drug classes which acts as an antiviral, anti-parasitic, anti-inflammatory and immunomodulators were considered for use in coronavirus. Finding an antiviral drug that diminishes the fatality rate in moderate to severe cases of coronavirus was the biggest demanding and difficult approach. Phase 3 trials drugs baloxavir and pimodivir for severe influenza were unsuccessful (NCT03684044 and NCT03376321)in treating COVID-19 disease. Similarly, hydroxychloroquine and lopinavir–ritonavir werenot successful in treating COVID-19 disease. However, it was demonstrated by WHO-led, open-label, randomized SOLIDARITY trial 3 that Remdesivir is effective only in hospitalized patients who requires supplemental oxygen of severe cases of COVID-19. Moreover, Remdesivir shortens the process of time to recovery and reduces the risk of progression of disease in patients supported with supplemental oxygen and have a higher risk of inflammation. Additionally, in the second wave of COVID—19, corticosteroid like dexamethasone is strongly recommended and effective in admitted patients supported with supplemental oxygen and in mechanical ventilation. In severe pneumonia-like cases of COVID-19, timely and prolonged administration of methylprednisolone (MP) significantly lowers the risk of mortality and decreases the ventilator dependence. Further, a recombinant humanized anti-interleukin-6 receptor monoclonal antibody, tocilizumab along with dexamethasone therapy effective in severe hypoxia COVID-19 patients. Other drugs like Favipiravir, Ribavirin, Ivermectin, and inhibitors of cytokines are equally efficacious and used in COVID-19 mild to moderate patients. According to current evidence, WHO recommends Ivermectin only to be used within clinical trials in COVID-19 patients until more data are available. Further, the antiviral drug, Favipiravir (Fabi-flu) fails viral replication and transcription by inhibiting the RNA-dependent RNA polymerase (RdRp). So far, scientists of various organizations worked day and night to develop relevant pharmacological therapeutics either through drug repositioning or CU interventions. Fig.3. illustrate how compassionate drugs are used based on the severity of the individual case.
The development of novel therapeutic interventions and vaccines for treatment within a short time span was one of the biggest challenges for the pharmaceutical industry as well as for the medical expert’s team because of unexpected outcomes with COVID-19 spread. Considering immediate unmet needs, all medical faculties,scientists, researchers, and government bodiesscannedthe existing drugs based on their symptomatology and their toxicity profile forrepurposing and explore their alternate use. The repurposed drugs were used in the line of compassionate use of drugs. Potential candidates for repurposing includeda variety of drug classes which acts as an antiviral, anti-parasitic, anti-inflammatory and immunomodulators were considered for use in coronavirus. Finding an antiviral drug that diminishes the fatality rate in moderate to severe cases of coronavirus was the biggest demanding and difficult approach. Phase 3 trials drugs baloxavir and pimodivir for severe influenza were unsuccessful (NCT03684044 and NCT03376321)in treating COVID-19 disease. Similarly, hydroxychloroquine and lopinavir–ritonavir werenot successful in treating COVID-19 disease. However, it was demonstrated by WHO-led, open-label, randomized SOLIDARITY trial 3 that Remdesivir is effective only in hospitalized patients who requires supplemental oxygen of severe cases of COVID-19. Moreover, Remdesivir shortens the process of time to recovery and reduces the risk of progression of disease in patients supported with supplemental oxygen and have a higher risk of inflammation. Additionally, in the second wave of COVID—19, corticosteroid like dexamethasone is strongly recommended and effective in admitted patients supported with supplemental oxygen and in mechanical ventilation. In severe pneumonia-like cases of COVID-19, timely and prolonged administration of methylprednisolone (MP) significantly lowers the risk of mortality and decreases the ventilator dependence. Further, a recombinant humanized anti-interleukin-6 receptor monoclonal antibody, tocilizumab along with dexamethasone therapy effective in severe hypoxia COVID-19 patients. Other drugs like Favipiravir, Ribavirin, Ivermectin, and inhibitors of cytokines are equally efficacious and used in COVID-19 mild to moderate patients. According to current evidence, WHO recommends Ivermectin only to be used within clinical trials in COVID-19 patients until more data are available. Further, the antiviral drug, Favipiravir (Fabi-flu) fails viral replication and transcription by inhibiting the RNA-dependent RNA polymerase (RdRp). So far, scientists of various organizations worked day and night to develop relevant pharmacological therapeutics either through drug repositioning or CU interventions. Fig.3. illustrate how compassionate drugs are used based on the severity of the individual case.

Fig.3. Compassionate drugs used in SARS-COV-2 (COVID-19) mild, moderate and severe cases based on their symptomatology.
2. Swine Flu
Swine flu is a respiratory disease caused by influenza A (H1N1 virus) in 2009-10 and affected rapidly worldwide. A lot of extensive investigations are to be taken to find the alternative approach to manage the outburst situation. To manage this situation, FDA allowed firstly peramivir, only for the hospitalized patients and for those who are at higher risk for death. Further, Committee for Medicinal Products for Human Use (CHMP) provided compassionate use of Tamiflu and zanamivir (i.v) for the severely sick patients for the treatment of H1N1 influenza.
3. Ebola Virus
Ebola virus is a viral hemorrhagic fever in humans caused by ebolaviruses in the year 2014-16, generally in West Africa. CU of investigational/experimental drugs in the management of ebolawasan imperative strategy for public health levels. There is only one hope for the individuals at that time who received drugs, despite the fact that its efficiency and adverse effects are mysterious. Experimental drugs such as GS-5734, REGN monoclonal antibody (combination Zmapp and mAb114) were firstly permitted by the Ethics Committee in Africa on an emergence basis. Close monitoring of patients during use of unapproved drugs was essential due to their unexpected outcome and unfamiliar adverse events. TherVSV-ZEBOV vaccine had been allowed for prophylaxis to persons who were suspected of close contact with infected patients.
4. Nipah Virus (NiV)
It is a zoonotic virus of the familyParamyxoviridae, genus Henipavirus, which primarily spreads between animals to humans. Initially, monoclonal antibody (m102.4) under investigation completed only phase-1 trials had been allowed for compassionate use basis. Later, Remdesivir was used as post-exposure prophylaxis due to its antiviral activity and might be corresponding to immunotherapeutic agents. In addition, Ribavirin wass also used to treat a small number of individuals, but their efficacy against NiVwas still not clear.
5. Multidrug Resistant Tuberculosis (MDR-TB)
Nearly, about last 5 decades of treating tuberculosis with the same drugs (first (Isoniazid,Rifampicin)& second-line injectables drugs) causing resistance to causative organisms (Mycobacterium tubercular). To address this situation, Bedaquiline was developed by Janssen and used ona compassionate basis when the drug was in phase 2b trial. Later, it was approved by US FDA in 2012 for the effective antitubercular drug. Further, another drug Delamanid, by Otsuka Pharmaceuticals, another class of drug was available along with some other antitubercular regimen (bedaquiline) to explore its efficacy for the treatment of pulmonary MDR-TB.
6. Human Immunodeficiency Virus (HIV)
At the time of the HIV epidemic, “Glaxo-Wellcome” a pharmaceutical company donated their unapproved antiretroviral drugs Zidovudine to many patientswhen it was undergoing clinical phase 3 trials. In addition, Ibalizumab, a humanized immunoglobulin G monoclonal antibody was approved for compassionate use after phase 1 and 2 trials. The drug showed its activity directed against T-cell receptor (CD4), thus suppressed replication of HIV. Another class of drug fostemsavir (GSK3684934; previously BMS-663068) was used on compassionate basis only for selected patients who didn’t respond to available drug therapy.
Ethical Challenges for Compassionate use
There is an intensifying challenge for the entire medical experts like physicians, health department, pharmaceutical industry, scientists from various organizations, ethics committee (IRB) and as well for patients to grant and right to use for investigational drugs, vaccines, biological and any medical devices which have not been approved by the regulatory authorities. The term compassionate use drugs are acceptable in those patients suffering from a life-threatening disease, so assessment regarding efficacy, safety, and toxicity could be uncertain. In addition, it may also a doubtful situation whether the use of compassionate drugs/products gives better output and would be adequate to save the life. Sometimes, compassionate use produces dismay to physicians and in many clinicians due to the expanded use of drugs in a large numbers of patients and requesting to diminish the supply of drugs for clinical research. Further, during placebo randomized controlled trials only a few patients would like to participateand create difficulty to analyze and interpret the safety and efficacy of experimental drugs without the placebo group. So from all of these challenges, there is necessitate to both regulatory bodies as well as to pharmaceutical companies to work mutually and broaden the range of spectrum of compassionate use of drugs for betterment to save the life of a needy patient.
Conclusion
The compassionate drug is preapproval access to drugs under regulatory bodies for patients suffering from long-lasting and life-debilitating situations. Several economically challenged countries suffer a lot for their survival due to unavailable treatment and make the patient in a hapless condition. There is a stupendous step proposed by the drug regulators in the amendment of New Drugs and Clinical Trial Rules (NDCT), 2019 will allow permitting for making provisions of investigational/experimental drugs to patients in emergencies and creates an immense hope to the patients and their families. Creating a regulatory framework and their outline facilitates the monitoring of experimental drugs that would help to ensure their safety along with the welfare of the patients. But there are certain challenges faced by pharmaceutical industries, physicians and research organizations for compassionate use of drugs. Considering all the facts and challenges together there is a need to work in partnership with academicians, researchers, physicians, and with the pharmaceuticals industries for compassionate use of drugs with clear and sturdy guidelines.
(The authors are Dean Academics, and Research Scholar, DPSRU New Delhi)

