Docking to Dwelling: Definite Drug the treatment of COVID-19

There is a good news for those who are looking for new drug for treatment of COVID-19. Thanks to the researchers/scientists of Delhi Pharmaceutical Sciences & Research University (DPSRU) who have achieved a milestone by inventing new Corona drug. DPSRU is one of premier Pharmaceutical Sciences & Research University of India. Double Helical feels pride to break such mind blowing and very informative story…
By DR. Ramesh K Goyal and DR. Subbu Apparsundaram and the Team Delhi Pharmaceutical Sciences & Research University
With the first case of corona reported from Wuhan, China on 31st December, there has been unprecedented outbreak of coronavirus disease (COVID-19). After over one year, the cases world-wide have not given satisfactory relief to be called as ‘Control over the Disease’. Still when this article is written, a total of about 128 million cases have appeared with over 21 million active cases and 2 million deaths world-wide (216 countries affected). Daily even today half a million new cases appear from 155 countries and out of them daily over 1000 deaths reported from 56 countries. Even new deaths are reported from over 100 countries.
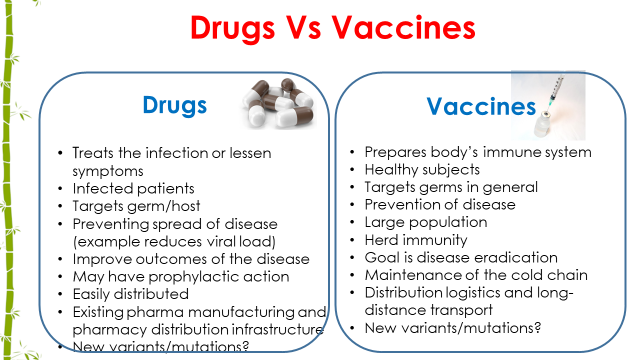
In the beginning the case fatality ratio was 5-10%, it is a bit consolation that it has been reduced to about 1-3 %. One of the major breakthrough has been the availability of vaccines. Lockdown, curfews, usage of masks and social distancing has given some rescue for the spread of the disease. With vaccines it was hoped that herd immunity will come and appearance of cases will go down. However, the appearance of the second wave all across the world in different countries across the world puts a question of early success of the vaccination. From all these facts and figures, the only answer is that “Development of effective medicine is the only answer.The following comparison speaks then same.
Earlier Strategies for Drug Discovery
In the beginning there was noWHO and FDA approved Drugs or vaccineavailable for COVID -19which to a great extent hold today the same. There has been an impatient bustling need to have a drug for the treatment. With the announcement of possibility of 4hydroxyquinoline last year, being effective in COVID-19, the is sudden upsurge of ‘repurposing of drugs’ as one of the strategies for the discovery of drug in COVOID-19. It was noticed that as on05 April 2020 for COVID-19there are 282 clinical trials going on with the ‘drug repurposing’ as the strategy. We reviewed various clinical trials from www.clinicaltrials.gov. It was noticed that apart from chloroquine and hydroxychloroquine, various the monoclonal antibody, corticosteroids, antibiotics, and many of the known antiviral drugs like arbidol, remdesivir, favipiravir, lopinavir, ritonavir, oseltamivirhave been taken for the clinical trials in various countries.WHO also came up with an initiative known as Multi-country ‘Solidarity Trial’for developing a potential drug or therapy against COVID19.With drug repurposing as one of the strategies with guidelines and strategies framed by the WHO formed an IPC(Infection Prevention and Control) for monitoring the widespread of this COVID-19 across the world.
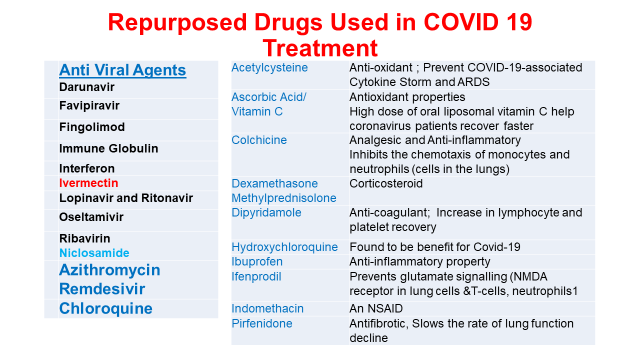 Considering previous viral infections Middle East Respiratory Syndrome (MERS-CoV) and severe acute respiratory syndrome (SARS-CoV) and the epidemics have occurred in the past decade, the first set of trials for the discovery of new drugs was to repurpose of antiviral drugs, used in various retroviruses i.e. for MERS, SARS, dengue, AIDS etc. In addition to antiviral drugs, anti-retrovirals, antimalarial drugs, antibiotics and antiparasitic, nonspecific anti-inflammatory and immunosuppressive drugs, kinase inhibitors, monoclonal antibodies and other miscellaneous drugs utilized for the design of new drug and vaccines against coronavirus disease. Remdesivir, a nucleotide analog is a broad-spectrum antiviral prodrug with effective in vitro antiviral activity against different RNA viruses such as SARS-CoV, MERS-CoV, Hendra virus, Nipah virus (NiV), Marburg, Ebola virus (EBOV), respiratory syncytial virus (RSV) provided success to a great extent. But it was again not a clear drug. Similar was the case with Favipiravir, chemically known as 6-fluoro-3-hydroxy-2-pyrazine carboxamide, a pyrazine derivative that is used as an anti-viral agent for the treatment of influenza in Japan. Other drugs like Ribavirin, Arbidol, (Umifenovir), Oseltamivir, Bromhexine a transmembrane protease serine (TMPRSS2) inhibitor, Fingolimod (Gilenya) is a sphingosine-1-phosphate receptor (S1P receptor) could not come up as real as efficacious and safe drug for COVID-19.
Considering previous viral infections Middle East Respiratory Syndrome (MERS-CoV) and severe acute respiratory syndrome (SARS-CoV) and the epidemics have occurred in the past decade, the first set of trials for the discovery of new drugs was to repurpose of antiviral drugs, used in various retroviruses i.e. for MERS, SARS, dengue, AIDS etc. In addition to antiviral drugs, anti-retrovirals, antimalarial drugs, antibiotics and antiparasitic, nonspecific anti-inflammatory and immunosuppressive drugs, kinase inhibitors, monoclonal antibodies and other miscellaneous drugs utilized for the design of new drug and vaccines against coronavirus disease. Remdesivir, a nucleotide analog is a broad-spectrum antiviral prodrug with effective in vitro antiviral activity against different RNA viruses such as SARS-CoV, MERS-CoV, Hendra virus, Nipah virus (NiV), Marburg, Ebola virus (EBOV), respiratory syncytial virus (RSV) provided success to a great extent. But it was again not a clear drug. Similar was the case with Favipiravir, chemically known as 6-fluoro-3-hydroxy-2-pyrazine carboxamide, a pyrazine derivative that is used as an anti-viral agent for the treatment of influenza in Japan. Other drugs like Ribavirin, Arbidol, (Umifenovir), Oseltamivir, Bromhexine a transmembrane protease serine (TMPRSS2) inhibitor, Fingolimod (Gilenya) is a sphingosine-1-phosphate receptor (S1P receptor) could not come up as real as efficacious and safe drug for COVID-19.
 Chloroquine and Hydroxychloroquine are well known antimalarial drugs with some immunosuppressant effect on increasing immune factors. However, some reports have shown their antiviral action which occurs at ACE 2 cell entry-level by inhibiting glycosylation of host receptors, proteolytic processing, and endosomal acidification.When Hydroxychloroquinewas reported to be potent against SARS-CoV-2, other antimalarial drugs like mefloquine and amodiaquine were tried and were not found effective against SARS-COV-2. Later, Emodin, was reported to block SARS CoV spike protein with ACE2 in a dose-dependent manner and ACE-2 became a target focus..
Chloroquine and Hydroxychloroquine are well known antimalarial drugs with some immunosuppressant effect on increasing immune factors. However, some reports have shown their antiviral action which occurs at ACE 2 cell entry-level by inhibiting glycosylation of host receptors, proteolytic processing, and endosomal acidification.When Hydroxychloroquinewas reported to be potent against SARS-CoV-2, other antimalarial drugs like mefloquine and amodiaquine were tried and were not found effective against SARS-COV-2. Later, Emodin, was reported to block SARS CoV spike protein with ACE2 in a dose-dependent manner and ACE-2 became a target focus..
Soon excessive immune mechanism involvement COVID surfaced. This brought many immnunomodulators for repurposing. Baricitiniban approved for rheumatoid arthritis having inhibitory action on Janus kinase (JAK)1/JAK2,Imatinibmesylate and dasatinib, inhibitors of the kinase signaling pathway, Tramitinib, Selumetinib, a potent inhibitor of MEK, and many other came into the race. Tocilizumab however, got a greater success among thes. Surprisingly, simple Dexamethasome proved highly useful as per the reports from Italy
Apart from respiratory failure and immune or cytokine surges another clinical reports showed that in COVID-19 patients which are critically ill, the patients have high incidences of of Thromboembolism Haemostatic abnormalities include mild thrombocytopenia and increased D-dimer levels leading to death. The use of anti-coagulant drugs in COVID-19 patient became yet another strategy but only to the patients having higher D-dimer levels.
 Having understood, the pathophysiology coming into the forefront several scientists all across the world to tried to manage the COVID-19. Development of the vaccine and the development of antiviral drugs with drug repurposing strategy have been on the top. Based on the clinical symptoms reported from time to time and then clarity the of the pathophysiological consequences of COVID-19, the use of non-conventional antibiotics like azithromycin, steroids like dexamethasone & monoclonal antibodies for immunomodulation, anti-thrombotics or thrombolytics and certain food supplements were recommended by the statutory bodies in different countries.
Having understood, the pathophysiology coming into the forefront several scientists all across the world to tried to manage the COVID-19. Development of the vaccine and the development of antiviral drugs with drug repurposing strategy have been on the top. Based on the clinical symptoms reported from time to time and then clarity the of the pathophysiological consequences of COVID-19, the use of non-conventional antibiotics like azithromycin, steroids like dexamethasone & monoclonal antibodies for immunomodulation, anti-thrombotics or thrombolytics and certain food supplements were recommended by the statutory bodies in different countries.
Considering the rampant deaths reported worldwide due to novel corona virus (COVID-19) on one side, hypertension, diabetes and renal failure emerging as comorbidities with mortality risk due to respiratory failure on the other side. The link of these morbidities with renin angiotensin system (RAS) and angiotensin converting enzyme-2 (ACE-2) as the site of the multiplication of COVID-19 has widely been accepted. Based on the clinical reports out of the deaths and the prognosis of COVID-19, from several parts of the world, following are the major pathophysiological and molecular derangements that have drawn the attention of the scientists for the discovery of the medicine that can be used for the treatment of COVID 19.
1. Binding of the virus with ACE-2 receptors leading to Viral multiplication, Over expression of ACE2 and thereby a total derangement of RAS; immunostimulation, inflammation, Cytokine surge (increase in TNFa, IL1, IL6,IL10etc).
Inflammation in the alveoli and cell death in alveoli of lungs and causing Acute Respiratory Distress or syndrome
Thrombotic events and embolism
Binding with ORF8 proteins, leading to dissociation of iron from the 1-beta chain of hemoglobin getting attached to the surface glycoprotein porphyrin.
In the light of clinical picture of the COVID-19, we described it as the “Virus induced Cardiovascular Pulmonary Disease”. Delhi Pharmaceutical Sciences and Research University (DPSRU), is the first Pharmacy University of India, has been working for last one year with team members from Pharmacognosy and Pharmaceutical Chemistry Departments. In addition, we partnered with Remedium Therapeutics for development of a formulation with ACE-2 as the Target and then to have the clinical Trials. The formulation was expected to be innovative therapies from herbal resources treatment of COVID-19.
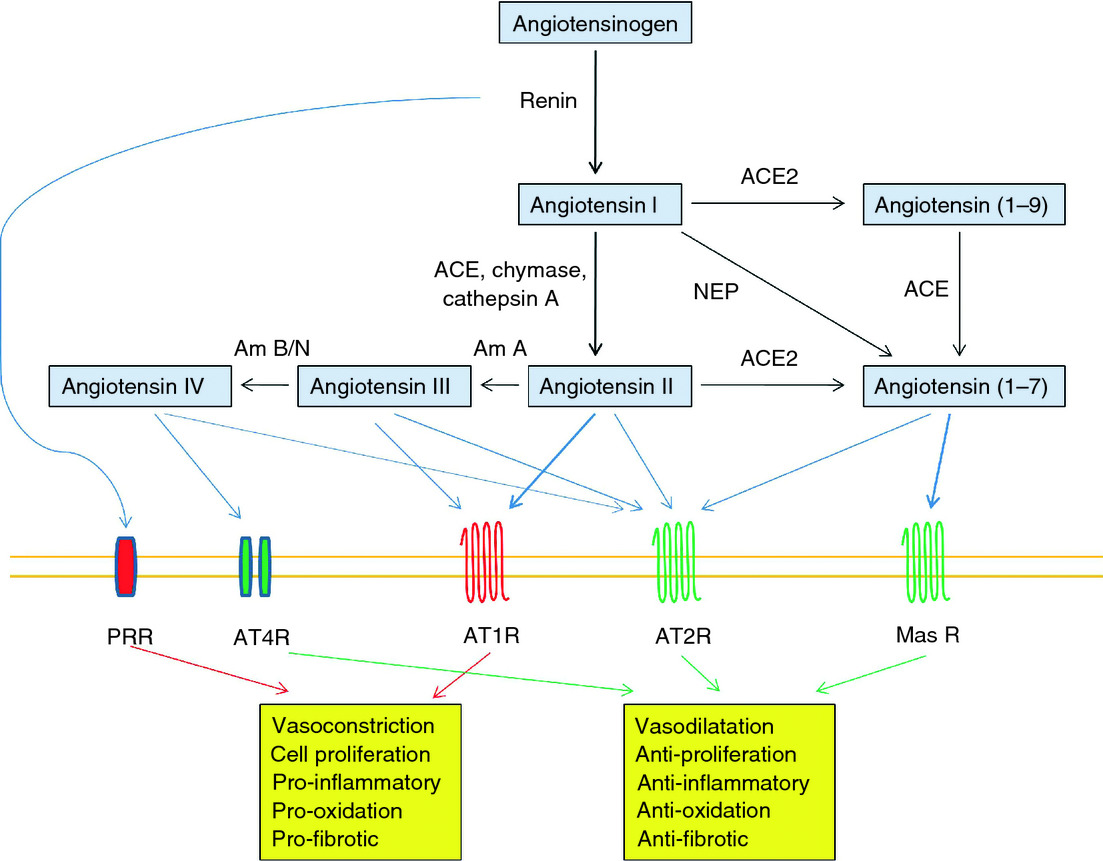
It is now well known that membrane-anchored ACE2, is well expressed in the epithelial cells of the lung, intestine, kidney, heart, and blood vessels express ACE2 and is also present in the oral and nasal mucosa, skin, lymph nodes, thymus, bone marrow, spleen, liver, and brain Due to its expression in a wide range of tissues and organs, ACE2 regulates the vasculature and inflammation, oxidative stress, fibrosis, and proliferation. ACE2 regulates Ang II action by hydrolyzing Ang II to Ang1-7. Ang1-7 exerts anti-inflammatory, antithrombotic, antihypertensive, antiarrhythmic, and cardioprotective action through G-protein-coupled Mas receptors (MasR). On the other hand, Ang II acting via AT1 receptors causes vasoconstriction, inflammation, cytokine secretion, thrombosis, endothelial and myocardial dysfunction, it also acts on AT2 receptors to reduce inflammation and oxidative stress. Thus, ACE and ACE2 arms of RAS counterbalance their actions fine-tuning many physiological function. Angiotensin receptor blockers (ARBs) and ACE inhibitors were investigated for the treatment of COVID-19, but it was a failure.
Various parts of the world have revealed that cardiovascular disturbances, including hypertension and thrombotic events, diabetes, and acute respiratory distress, are emerging comorbidities. Principal molecular targets for COVID-19 are host ACE2, viral RdRp, and Open Reading Frame (ORF8) proteins. Viral replication, cytokine surge, inflammation, apoptosis of Type 1 & 2 cells in alveoli, and dissociation of iron from hemoglobin involving porphyrin, and thereby the failure of internal respiration turned out to be the therapeutic targets for the management of COVID-19.
 Herbal Approach for the treatment of COVID-19
Herbal Approach for the treatment of COVID-19
Various herbal phytoconstituents (baicalin, glycyrrhizin, scutellarin, and hesperetin), are considered for COVID-19 due to their potential action on ACE2. The significant advantage of using herbs in viral respiratory infections is due to their potent anti-inflammatory and immunostimulatory action. Various edible plants have been reported to have a potential for the treatment of COVID-19 disease. Considering the pathogenesis of COVID-19, we identified some Indian medicinal plants with potent anti-inflammatory, immunomodulatory, and antiviral activities for developing potential treatment of COVID-19. We investigated potential chemical constituents from plants (Phytoconstituents).
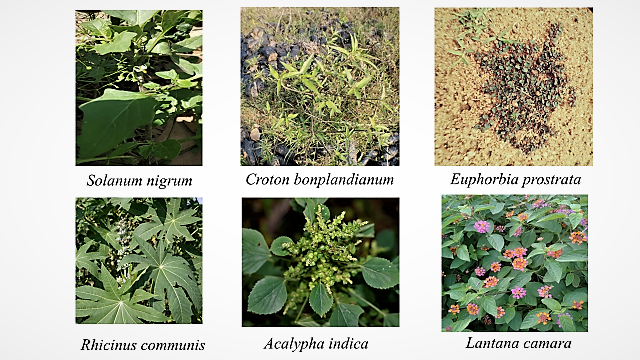
Several Rasayana botanicals described in Ayurveda are used in clinical practice for strengthening immunity. Among various Rasayana, we found Solanum nigrum (Makoya) and Euphorbia prostrata were found to be the most appropriate plants that can be used for the treatment of COVID-19. Both are reported to possess antiviral, immune-modulator and anti-inflammatory activities. In addition, they also have, other desirable effects that may be due to ACE2 or ORF. Solanum nigrum L. (Fam. Solanaceae) commonly called as Black Nightshade in English and Makoya in Hindi, grows in temperate climate zones and found throughout the country in dry parts. The drug “Kakamachi (whole plant)” of Ayurveda is used in Sotha (inflammation), Hrdroga (Heart disease), Jvara(fever), Prameha (Diabetes), Hikka (Hiccups). It is described as Kaphahara, Pittahara, Vatahara and Rasayana.Solanum nigrum possesses various compounds that are responsible for diverse activities. Euphorbia prostrata (Euphorbiaceae) is a small, prostrate, more or less pubescent annual herb found throughout India as a naturalized weed. It is commonly called as Dudhi in Hindi and Svaduparniksirni in Sanskrit.The Ayurvedic drug, Dugdhika, consist of whole plant and mainly used for Svasa (Dyspnea, bronchial Asthma), Prameha (Diabetes), Raktapitta (bleeding disorders), Raktarsa (Bleeding piles). In Ayurvedicit has been described as Kaphahara, Mutrala (diuretic), Hrdya (heart disease), Vistambhini (Anti-carminative), Grahi (anti-diarrhea), Malastambhaka etc.Solanum nigrum and Euphorbia prostrata possess various compounds that are responsible for diverse activities. Among various compounds reported we found that Solanine to have docking score of -9.424 with ACE2 and also the Rutin to have the docking score of -10.871. Similarly, from Euphorbia prostrata the various compounds (Apigenin: -6.204; Apigenin-7-glucoside: -8.06and Luteolin 7-O-glucoside: -9.297 with ACE2. Interestingly some compounds from Euphorbia prostrata showed very high binding with ORF 8 (Apigenin: -9.033, Apigenin-7-glucoside: -12.603, and Luteolin 7-O-glucoside: -13.06). Clinical reports of the patients and mortality cause analysis of the deceased patients from various parts of the world have revealed that cardiovascular disturbances including hypertension& thrombotic events, diabetes and acute respiratory distress are emerging comorbidities. Principal molecular links of these morbidities and COVID-19 prognosis emerged are angiotensin converting enzyme-2 (ACE2) and Open Reading Frame (ORF8). Viral multiplication, cytokine surge, inflammation, apoptosis of Type 1 & 2 cells in alveoli and dissociation of iron from the 1-beta chain of hemoglobin getting attached to the surface glycoprotein porphyrin and thereby failure of internal respiration. Preliminary work has been carried out on different plants reported in Ayurveda as the Rasayana, out of which two plants Solanum nigrum and Euphorbia prostrata, found in Aravalli Hills Biodiversity of Delhi as well available in Andaman & Nicobar Islands.
 Based on extensive work on certain plants reported in Ayurveda as the Rasayana, we found Solanum nigrum L. (family – Solanaceae) might be the most effective in COVID-19. Its phytoconstituents may have antiviral activity against SARS-CoV-2. Also, it may be effective against associated comorbidities, including respiratory failure and cardiovascular complications. The results were correlated with published pharmacological studies. Since, certain formulations of exclusive were available in India and this plant is an eatable vegetable in certain parts of the world, it was given to patient as add-on nutraceutical to some patients. They are now looking into the development of a phytopharmaceutical products containing single plant bioactive compounds that are quantitatively and qualitatively defined.
Based on extensive work on certain plants reported in Ayurveda as the Rasayana, we found Solanum nigrum L. (family – Solanaceae) might be the most effective in COVID-19. Its phytoconstituents may have antiviral activity against SARS-CoV-2. Also, it may be effective against associated comorbidities, including respiratory failure and cardiovascular complications. The results were correlated with published pharmacological studies. Since, certain formulations of exclusive were available in India and this plant is an eatable vegetable in certain parts of the world, it was given to patient as add-on nutraceutical to some patients. They are now looking into the development of a phytopharmaceutical products containing single plant bioactive compounds that are quantitatively and qualitatively defined.
 Out of various plants identified, we shortlisted Solanum nigrum, Euphorbia prostrata, Acalyphaindica, Lantana camara and Ricinuscommunisas potential plants. A literature searches to identify the phytoconstituents of these plants revealed that they contain flavonoids, flavonoid glycosides., triterpenoids, steroidal glycoalkaloids and other compounds of different classes. Flavonoids, in particularly, luteolin, quercetin, apigenin, amentoflavone, epigallocatechin (EGC), epigallocatechin gallate (EGCG), gallocatechin gallate (GCG) and kaempferol have been reported to inhibit the proteolytic activity of SARS-CoV 3CLproand 3a channel protein of coronavirus. We docked certain compounds for the ACE2 activity10. Results of docking studies it was found that many compounds from Solanumnigram like rutin, verbascoside, and hesperidin bound tightly at the active site of ACE2.. The compound rutin (docking score: -11.5 kcal/mol) well occupied the receptor cavity through hydrogen bonding with Ala348, Asp350, Phe390, Ser44, Ser47 Arg393, Asn394 and verbascoside (docking score: –10.2 kcal/mol) showed hydrogen bonding interaction with Ala348, Asp350, Asp382, Phe390, Glu375, His37, Asn394 and Glu402, whereas hesperidin (docking score: –9.5 kcal/mol) showed hydrogen bonding interaction with Ala348, Asp350, Trp69 and Tyr385.
Out of various plants identified, we shortlisted Solanum nigrum, Euphorbia prostrata, Acalyphaindica, Lantana camara and Ricinuscommunisas potential plants. A literature searches to identify the phytoconstituents of these plants revealed that they contain flavonoids, flavonoid glycosides., triterpenoids, steroidal glycoalkaloids and other compounds of different classes. Flavonoids, in particularly, luteolin, quercetin, apigenin, amentoflavone, epigallocatechin (EGC), epigallocatechin gallate (EGCG), gallocatechin gallate (GCG) and kaempferol have been reported to inhibit the proteolytic activity of SARS-CoV 3CLproand 3a channel protein of coronavirus. We docked certain compounds for the ACE2 activity10. Results of docking studies it was found that many compounds from Solanumnigram like rutin, verbascoside, and hesperidin bound tightly at the active site of ACE2.. The compound rutin (docking score: -11.5 kcal/mol) well occupied the receptor cavity through hydrogen bonding with Ala348, Asp350, Phe390, Ser44, Ser47 Arg393, Asn394 and verbascoside (docking score: –10.2 kcal/mol) showed hydrogen bonding interaction with Ala348, Asp350, Asp382, Phe390, Glu375, His37, Asn394 and Glu402, whereas hesperidin (docking score: –9.5 kcal/mol) showed hydrogen bonding interaction with Ala348, Asp350, Trp69 and Tyr385.
We developed a phytopharmaceutical formulation, and validated its pharmacological activities, including antiviral, anti-inflammatory, and immunomodulatory actions. A purified extract of Solanum nigrum with four active phytoconstituents (Solanine, Solamargine, Rutin and Luteolin) was used for pharmacological evaluation and validation of the beneficial activities on ACE2 with its receptors, AT1 and AT2 receptors as well as ORF8 as the principle targets based on our preliminary docking studies. The extract with phytoconstituents was tested in vitro viral cell lines at the University of Missouri, USA.
Most patients turned COVID negative (as per the qRTPCR) report and there has been a distinct improvement of the WHO Ordinal scale by 2+ . It is expected that the herbal formulation will soon be released after due approvals from the Govt. bodies.
With inputs from
Prof. C. R. Babu (Aravalli Biodiversity Park, New Delhi),
Pat Krishnan (Remedium, USA),
Dr. Bhoomika Patel (Nirma University),
Prof. Rajiv Tonk (DPSRU),
Dr Mahaveer Dhobi (DPSRU),
Dr. Jaseela Majeed (DPSRU),
Dr. Kalicharan Sharma (DPSRU) and Dr. Jammi Narsimhan
(Jammi Pharmaceuticals)

