Mission TB FREE India
Elimination of TB means stopping transmission. That is reach every person suspected to have tuberculosis, get diagnosis confirmed, and if positive put on appropriate treatment and help to complete the therapy. Given the difficult terrain and hard to reach population, more resources would be needed…
By Amresh Kumar Tiwary
Our Prime Minister Narendra Modi’s statement to India is determined to address the challenge of TB in mission mode. He is confident that India can be free of TB by 2025. As he recently inaugurated the Delhi End TB Summit and launched the TB Free India Campaign.
In addition to the well-known risk factors contributing to the rise of tuberculosis cases in India, like human immunodeficiency virus/acquired immune deficiency syndrome (HIV/AIDS), poor nutritional status, and young age, realization that other emerging factors like diabetes mellitus, indoor air pollution, alcohol abuse, tobacco smoke are also fuelling the epidemic, is adding more complexities to tuberculosis control and making the task onerous. Individually and in combination risk factors tend to increase the burden two to three times.
 Unless addressed concurrently these numbers are likely to overwhelm the tuberculosis control programme and annul its efforts. The eight states of the North Eastern region characterized by hilly, forested area, sparsely inhabited mainly by tribal populations also share high prevalence of emerging risk factors for tuberculosis. India has set itself a target of elimination of tuberculosis by 2025.
Unless addressed concurrently these numbers are likely to overwhelm the tuberculosis control programme and annul its efforts. The eight states of the North Eastern region characterized by hilly, forested area, sparsely inhabited mainly by tribal populations also share high prevalence of emerging risk factors for tuberculosis. India has set itself a target of elimination of tuberculosis by 2025.
To achieve the target in the North Eastern states special resources would be needed to be put in place for controlling these risk factors as well. A comprehensive integrated approach taking help of other departments in health sector and beyond is critical.
There are two risk factors like diabetes mellitus and tobacco smoking which impact a larger section of North-Eastern population and accelerate progression of tuberculosis disease. Some of the states here have highest prevalence of HIV in India, notably Manipur (1.15%), Nagaland and Mizoram (0.7–0.8%). India has an average of 0.26%. People with HIV have a 20–30 times higher risk of developing active tuberculosis, which is more of the extra pulmonary type and throws up challenges of diagnosis and management.
Tobacco consumption is highest in this region of the country. On an average people in NE smoke more tobacco than rest of India. Mizoram and Meghalaya have a prevalence of over 60%, Tripura follows at 40%. India’s average is around 26%. One in four Mizo women smokes, whereas average for India is 1 per hundred. Smokers are two-three times at higher risk of developing tuberculosis than non-smokers. The disease is more severe. A regular smoker has twice the risk of getting the disease again, recurrences are more often. If an HIV infected individual also smokes, the risk increases three folds.
Diabetes is the third risk factor. Results of an India-wide show high prevalence of pre-diabetes especially amongst the urban poor in the states of Arunachal Pradesh, Manipur, and Meghalaya which is of major concern.3 Diabetes again increases the risk of tuberculosis to three folds and the risk of multi drug resistant (MDR) among diabetics who get TB is 2–8 times higher. The progression of the disease is rapid. And it develops more frequently when the diabetes control is poor.
What does all this mean to the tuberculosis elimination programme? Elimination of TB means stopping transmission. That is reach every person suspected to have tuberculosis, get diagnosis confirmed, and if positive put on appropriate treatment and help to complete the therapy. Given the difficult terrain and hard to reach population, more resources would be needed. Health workers may have to travel long distances to bring one patient under treatment successfully. Finding TB cases is critical.
Modelling studies have shown that if the case detection is increased by 25%, it can translate in to about 40% reduction in mortality, the prevalence decreases by about 30% and the reduction of incidence cases is by more than 20% in 10 years.4 For persons with chest symptoms, sputum examination for acid-fast bacilli (AFB) is the recommended test. Acid fast staining of sputum for AFB performs poorly as a screening test. Its sensitivity is poor. The cartridges based nucleic acid amplification test (Cartridge Based Nucleic Acid Amplification test, CB-NAAT) is now available at the district level as it needs a controlled temperature and dust free environment.
A nucleic acid amplification test (True Nat MTB), a chip based test has been developed by an Indian company. It is reported to have good sensitivity and specificity.5 It has recently been validated in 100 designated microscopy centres in 50 districts in 10 states in which 18,000 samples have been tested. This battery operated test takes around an hour to give the result whether a sputum sample is positive for TB, for positive samples resistance can be determined in another hour’s time. It does not require dust proof air-conditioned environment. It is projected as a test to be used at primary health centre (PHC) level. If it is found to have an acceptable sensitivity and specificity, this test should be deployed in the NE states on a priority basis.
Relying on symptoms-screen alone may be contributing to delayed diagnosis of tuberculosis. Using chest X-rays (CXR) as a pre-screen test can reduce numbers needed to test for each case of tuberculosis. Abnormal CXRs could, therefore, be key to active case finding by identifying cases that otherwise would have not have been diagnosed by conventional, passive case finding,
Today, CXR is becoming more accessible in remote settings due to technological advances such as digital imaging instead of film-processed images. The sensitivity of CXR has been shown to increase if computer-aided diagnosis (CAD) software is used to analyse digital images. It gives a probability percentage consistent with TB. It could possibly be used as a ‘filter’ in TB screening to identify that gets tested by CB NAAT (GeneXpert). We need a locally available and economic version of the CAD4TB which would help in improving diagnosis especially in areas where a radiologist is not available to interpret the CXR. Diabetes triples the risk for active tuberculosis, thus the increasing burden of type 2 diabetes will further burden the TB elimination programme. An epidemiological model in India indicates that diabetes mellitus may account for 15% of TB cases. The International Diabetes Federation has predicted an increase in diabetes prevalence to 10% world wide by 2035. Modelling exercises have predicted that if such an increase does happen it could undercut the decrease in new cases of tuberculosis by about 3%. Some believe that increase in the prevalence of diabetes in India has contributed in part to a negligible reduction in new cases of tuberculosis between 1988 and 2008.
Diabetic tuberculosis patients have a higher risk of treatment failure, death, and recurrent tuberculosis as compared to non-diabetic tuberculosis patient. Poorly controlled diabetes increase the risk of tuberculosis and leads to unfavourable tuberculosis treatment outcomes. Researchers have long known that diabetes patients have higher blood sugar levels making their disease difficult to control and putting them at greater risk of developing complications. A bidirectional screening for tuberculosis and diabetes mellitus at hospital and community level has been shown to be feasible and effective.10,11 Such a screening should be piloted at hospital and community level and scaled-up. This presents a unique opportunity to capture persons presenting with either of these two conditions as potential targets for screening and treatment. Patients with diabetes often present with atypical symptoms and pose hurdles in diagnosing tuberculosis. Clinical management of patients with both diseases can be difficult. Tuberculosis patients with diabetes have a lower concentration of tuberculosis drugs and a higher of drug toxicity than tuberculosis patients without diabetes. Besides drug treatments for tuberculosis and diabetes, other interventions, such as education, intensive monitoring, and lifestyle interventions, might be needed, especially for patients with newly diagnosed diabetes or those who need insulin.
Modelling study analysed the potential effect of diabetes on tuberculosis epidemiology in 13 countries with high TB burden. The study estimated the tuberculosis burden that can be reduced by alternative scenarios of diabetes lowering the prevalence of diabetes by an absolute level of 6.6–13.8% could accelerate the decline of tuberculosis incidence by an absolute level of 11.5–25.2% and tuberculosis mortality by 8.7–19.4%. If interventions reduce diabetes incidence by 35% by 2025, 7.8 million tuberculosis cases and 1.5 million tuberculosis deaths could be averted by 2035.
The evidence for an regular tobacco smoking increases risk of TB in active smokers is well established. There is also some evidence that second hand smoking (passive smoking) is a risk factor for developing tuberculosis especially in children 0–5 years. When exposed to second hand smoke, household/ environmental factors (crowding, biomass fuel burning) may increase risk for developing tuberculosis. In addition, smoking has been associated with cavitary lesions, bacillary load, smear conversion delay, and high risk of reactivation and death during or after treatment. Smoking rates are high among men in North Eastern states, and, together with rising rates of diabetes, the risk of progression to tuberculosis disease will also increase.
Interventions like smoking cessation and early screening for tuberculosis can be advocated, but the impact of interventions in reducing TB risk remains negligible at population level. Both active and passive smoking increase susceptibility to TB infection, progression to active TB disease and the risk of adverse anti-TB treatment outcomes. Systematic reviews suggest that the risk of TB disease among smokers is increased two to threefold compare with people who have never smoked. Tobacco control and smoking cessation among people with diabetes and tuberculosis can therefore play an important role in limiting the burden of TB. It is also known that diabetic smokers have more than 5-fold increased risk of pretreatment positive smears than do non-diabetic non-smokers.
This is a remarkable joint effect of diabetes and smoking that increased risk of tuberculosis transmission. Against the background of risk factors fuelling the epidemic of tuberculosis in India, a critical assessment of the tuberculosis control programme (like strengths, weaknesses, opportunities and threats (SWOT) analysis) especially in the North- Eastern region would be helpful in identifying the areas that need strengthening to deal with these risk factors, and the resultant possible increase in number of active tuberculosis patients. From a health systems point of view, issues such as delays in diagnosis, initiation of appropriate treatment and its successful completion would be crucial. Experience from the combating combined HIV/TB disease would be helpful. But more operational research would be needed to tackle diabetes and tobacco smoking.
The Revised National Tuberculosis Control Program (RNTCP) would need to solicit assistance from other programmes within and outside the health sector to develop integrated comprehensive approach in meeting the targets of tuberculosis elimination in the North Eastern region.
What is Tuberculosis?
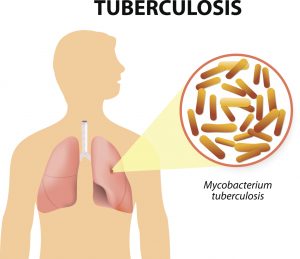
Tuberculosis (TB) is a multisystemic infectious disease caused by Mycobacterium tuberculosis (or TB), a rod-shaped bacterium. According to the World Health Organization, tuberculosis is the most common cause of infectious disease-related mortality worldwide (about 10 million people worldwide were sick with TB in 2015, and about 1.8 million people died from TB worldwide in 2015.
HIV-associated TB infections are a leading cause of death in HIV patients. TB symptoms can span such a wide range that TB is termed the “great imitator” by many who study infectious diseases because TB symptoms can mimic many different diseases. Additional terms are used to describe TB. The terms include consumption, Pott’s disease, active, latent, pulmonary, cutaneous, and others (see the following section), and they appear in both medical and nonmedical publications. In most instances, the different terms refer to a specific type of TB with some unique symptoms or findings.
TB has likely been infecting humans for many centuries; evidence of TB infections has been found in cadavers that date back to about 8000 BC. The Greeks termed it as a wasting away disease (phthisis). For many European countries, TB caused death in about 25% of adults and was the leading cause of death in the U.S. until the early 1900s. Robert Koch discovered TB’s cause, Mycobacterium tuberculosis, in 1882. With increased understanding of TB, public health initiatives, treatment methods like isolation of patients (quarantine), and the development of drugs to treat TB, the incidence of the disease, especially in developed countries, has been markedly reduced. However, the CDC estimates one-third of the world’s population is infected with TB with about 1.8 million deaths per year. About 60% of all TB-infected people are located in India, Indonesia, China, Nigeria, Pakistan, and South Africa.
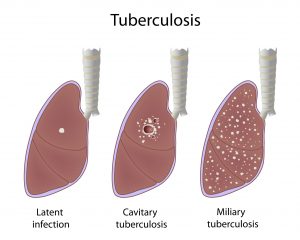
There are many types of tuberculosis, but the main two types are termed either active or latent tuberculosis infection. Active TB is when the disease is actively producing symptoms and can be transmitted to other people; latent disease is when the person is infected with Mycobacterium tuberculosis bacteria, but the bacteria are not producing symptoms (usually due to the body’s immune system suppressing the bacterial growth and spread) and have no TB bacteria in the sputum.
People with latent TB usually cannot transfer Mycobacterium tuberculosis bacteria to others unless the immune system fails; the failure causes reactivation (bacterial growth is no longer suppressed) that results in active TB so the person becomes contagious. Latent TB resembles chickenpox infection that goes dormant and may reactivate years later.
Many other types of TB exist in either the active or latent form. These types are named for the signs and for the body systems Mycobacterium tuberculosis preferentially infect, and these infection types vary from person to person. Consequently, pulmonary tuberculosis mainly infects the pulmonary system, cutaneous TB has skin symptoms, while miliary TB describes widespread small infected sites (lesions or granulomas about 1 mm-5 mm) found throughout body organs. It is not uncommon for some people to develop more than one type of active TB.
The cause of TB is infection of human tissue by the bacterium Mycobacterium tuberculosis (mycobacteria or TB). These bacteria are slow growing, aerobic, and can grow within body cells (an intracellular parasitic bacterium). Its unique cell wall helps protect it from the body’s defenses and gives mycobacteria the ability to retain certain dyes like fuchsin (a reddish dye) after an acid rinse that rarely happens with other bacterial, fungal, or parasitic genera.
Mycobacteria that escape destruction by body defenses may be spread by blood or lymphatic pathways to most organs, with preference to those that oxygenate well (lungs, kidneys, and bones, for example). Typical TB lesions, termed granulomas, usually consist of a central necrotic area, then a zone with macrophages, giant Langerhans cells and lymphocytes that become surrounded by immature macrophages, plasma cells, and more lymphocytes. These granulomas also contain mycobacteria. In latent infections, a fibrous capsule usually surrounds the granulomas, and in some people, the granulomas calcify, but if the immune defenses fail initially or at a later time (reactivate), the bacteria continue to spread and disrupt organ functions.
for some people to develop more than one type of active TB. More types will be listed in the symptoms and signs section below.
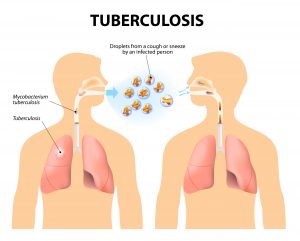
TB is contagious and can be spread to others by airborne droplets during sneezing, coughing, and contact with sputum, so you can get the disease by close contact with infected people; outbreaks occur in crowded conditions. The incubation period may vary from about two to 12 weeks. A person may remain contagious for a long time (as long as viable TB bacteria are present in sputum) and can remain contagious until they have been on appropriate therapy for several weeks. However, some people may be infected but suppress the infection and develop symptoms years later; some never develop symptoms or become contagious.
Because TB may occur as either a latent or active form, the definitive diagnosis of active tuberculosis depends on the culture of mycobacteria from sputum or tissue biopsy. However, it may take weeks for these slow-growing bacteria to grow on specialized media. Since patients with latent TB do not require isolation or immediate drug therapy, it is useful to determine if a person is either not infected, has a latent infection, or is actively infected with transmissible TB bacteria. Consequently, doctors needed a presumptive test(s) that could reasonably assure that the person was infected or not so therapy could begin. After getting a patient’s history and physical exam data, the next usual test is the skin test (termed the Mantoux tuberculin skin test or the tuberculin skin test or TST). The test involves injecting tuberculin (an extract made from killed mycobacteria) into the skin. In about 48-72 hours, the skin is examined for induration (swelling) by a qualified person; a positive test (induration) strongly suggests the patient has either been exposed to live mycobacteria or is actively infected (or had been vaccinated); no induration suggests the person tests negative for TB. This test can have false-positive results (especially in individuals vaccinated for TB with the BCG vaccine). False negative results can be caused by patients who are immunocompromised.
Another test, IGRA (interferon-gamma release assays) can measure the immune response to Mycobacterium tuberculosis. Other quick tests are useful; chest X-rays can give evidence of lung infection while a sputum smear stained with certain dyes that are retained mainly (but not exclusively) by mycobacteria can show the presence of the bacterium. These tests, when examined by a doctor, are useful in establishing a presumptive diagnosis of either latent or active TB, and most doctors will initiate treatment based on their judgment of these tests. In addition, some of these tests are useful in the U.S. and elsewhere only in people who are not vaccinated with a TB vaccine (see below) but are less useful in vaccinated people. For some patients, culture studies still should be completed to determine the drug susceptibility of an infecting TB strain.
Other tests have been developed. For example, a PCR test (polymerase chain reaction) to detect TB antigens and the LED-FM microscopic technique to identify TB organisms with microscopy may be used. Two other TB blood tests (also called interferon-gamma release assays or IGRAs) have been approved by the FDA and measure how strongly the body’s immune system reacts to TB bacteria. IGRAs are recommended in testing patients who have been vaccinated against TB (see prevention section below). People with positive symptoms, positive blood tests, sputum smear, or culture positive are considered infected with TB and contagious (active TB).
Treatment
The treatment for TB depends on the type of TB infection and drug sensitivity of the mycobacteria. For latent TB, three anti-TB drugs are used in four different recommended schedules. The drugs are isoniazid (INH), rifampin (RIF; Rifadin), and rifapentine (RPT; Priftin) and the CDC’s four recommended schedules are below and are chosen by the treating doctor based on the patients overall health and type of TB the patient was likely exposed to The most current treatment guidelines need to be reviewed and correlated to the patient’s specific condition and circumstances before any treatment is started.
Treatment of drug-resistant and multidrug-resistant tuberculosis TB can be difficult. Patients with these infections are recommended by the CDC to involve infectious-disease specialists as there are multiple approaches that involve other anti-TB drugs and variable treatment schedules that can be used. In addition, there are new drugs and treatment schedules being developed and approved by the FDA.
The infectious-disease consultant may be aware of these newest treatments that may benefit specific patients. For example, bedaquiline (Sirturo) has been approved for treatment of MDR TB, and research with an antimicrobial drug, moxifloxacin, suggests it may help in treatment protocols. Some side effects of treatment may include: Loss of appetite, Nausea and/or vomiting, Jaundice, Paresthesia, Bruise formation, bleeding and Vision changes.
Patients are urged to see their doctor if any side effects occur. In some patients, the lung destruction may be severe and the only treatment left may be surgical resection of the diseased lung tissue. Medications are needed for TB treatment. Home remedies will not treat TB but at best may help reduce symptoms. Home remedies may include milk, pineapple, Indian gooseberry, bananas, and many others. Patients should discuss these remedies with their doctors before use.

