Health Economics of Smoking Cessation Interventions

Smoking is known to be a key modifiable risk factor for a number of non-communicable diseases (NCDs) such as cancer, cardiovascular disease (CVD) and chronic respiratory conditions. Smoking also reduces life expectancy by 10 years…
BY Dr. Subhrojyoti Bhowmick
Cigarette smoking is rampant across India, with 14% of the population reported to smoke cigarettes, and a study has reported that 28.5% of Indians are ever-smokers. India has over 100 million adult smokers, which is the highest in the world after China.
Nearly a quarter of the male population smokes cigarettes, with a higher prevalence among the elderly, widowed, alcohol consumers, manual labourers and those with lower education end economic status. Similarly, the Global Adults Tobacco Survey-India (GATS-India) reported a higher prevalence of smoking among males, illiterate individuals, those from poor households and rural areas. The average number of cigarettes smoked per day is reported to be 7 per day among women and 6.1 per day among men.
 Disease burden
Disease burden
It is alarming to note that 14% of ever-smokers in India show some form of respiratory illness. The burden of tobacco-related cancers in India was estimated 366,000 in 2015 and
is estimated to increase by nearly 40% by the year 2050, with an estimated burden of 508,000.There is also significant cardiovascular disease (CVD) burden due to
smoking, with most CVD deaths attributed to tobacco smoking. Five percent of deaths among Indian women and 20% of deaths among Indian men are attributed to smoking. In comparison to never smokers, current smokers had an age adjusted relative risk (RR) of 4.6 for myocardial infarction. In addition, persons consuming more than 10 cigarettes per day (median 15 cigarettes/day) had an RR of 7.3 in comparison to never smokers.
Direct and Indirect cost impact
Using information from the National Sample Survey and the Global Adult Tobacco Survey, it was determined that the economic costs of tobacco use amount to approximately 1.04% of India’s GDP, while direct health expenditure for the treatment of tobacco-related diseases accounted for 5.3% of total private and public health expenditures.
The total economic costs attributable to tobacco use from all diseases and deaths in India in the year 2017-18 for persons aged 35 years or older amounted to INR 1,773.4 billion (US$ 27.5 billion), of which 22% was direct and 78% was indirect cost. Smoking contributed 74% of the total cost. A majority of the costs (93.3%) were borne by those in the age group 35-69, while those aged 70 and above shared the remaining 6.7%.The costs of premature mortality alone were 75% of the total economic cost. The total cost of premature mortality due to tobacco use across all age groups was INR 1324.5 billion as shown in Table 4. Costs from smoking accounted for 76% (INR 1006.6 billion) of the cost of premature mortality attributed to tobacco use.
The estimated total economic costs attributable to tobacco use is about 1.04% of the GDP in the year 2017-18 while the total excise tax collected on tobacco products in the previous year amounted to only about 12.2% of this cost. In other words, for every INR 100 that is received as excise taxes from tobacco products, INR 816 of costs is imposed on society through the consumption of tobacco.
Impact of smoking on health
According to the World Health Organization (WHO), over 5 million people die each year due to diseases associated with smoking, such as cancer, heart disease, liver disease, and stroke. In fact, nearly 16% of all NCD deaths and 1-0-30% of all cardiovascular deaths are linked to smoking. It is estimated that by the year 2030, there would be 8 million deaths attributable to tobacco smoking. Even exposure to second-hand smoke (SHS) increases the risk of developing and progression of atherosclerosis.
The health risks of tobacco smoking have been demonstrated in several studies, indicating a 2-3 fold higher relative risk of coronary heart disease (CHD), 1.5 times for stroke, 1.4 times for chronic obstructive pulmonary disease (COPD) and 12 fold risks for lung cancer. The risks are similar in men and women, but are higher for younger individuals.
Current smoking cessation aids
Role of smoking cessation
Smoking is a leading cause of preventable morbidity and mortality across the globe.The number of smokers is expected to be in excess of 1.6 billion by the year 2030, despite smoking cessation interventions and increasing awareness of the dangers of smoking. Smoking cessation has a major health impact. Smoking cessation is associated with a lower risk of CVD within 5 years of smoking cessation. Smoking cessation is associated with an 18% lower risk of all cancer, 26% lower risk of smoking-related cancer, and 45% lower risk of lung cancer compared to heavy smokers. Furthermore, smokers who quit before the age of 35 years have a life expectancy similar to individuals who have never smoked.
Studies in USA report that 66% of adult smokers express a desire to quit smoking, but only 50% try to quit each year. Alarmingly, less than 10% of smokers who try to quit succeed in quitting for 6 months or longer. Furthermore, 75% of those who attempt to quit smoking by themselves relapse within the first week. There is evidence that receiving medical advice to quit smoking leads to 1-year abstinence rates of 5-10%, which could translate to public health benefits. There is a need for implementation of smoking cessation strategies using a variety of available non-pharmacological (behavioural counselling) and pharmacological means.
Nicotine replacement therapy as part of smoking cessation: Rationale
Nicotine replacement therapy (NRT) involves the controlled administration of nicotine, thus partially replacing the nicotine previously obtained from tobacco use. NRT stimulates nicotine receptors leading to immediate removal of the craving to smoke as well as symptoms of withdrawal. Furthermore, a slower effect of NRT that ultimately reduces tobacco dependence is the reduction in the number of nicotine receptors. NRT is delivered at a lower dose and with slower nicotine pharmacokinetics than cigarettes, which allows for lower levels of nicotine, but for prolonged periods of time. This reduces not only the rewarding effects but also withdrawal symptoms. NRT addresses physical nicotine dependence without exposing the person who is trying to quit to the toxic constituents generated by combustion or other additives.

A number of NRT products are available, including nicotine gum, transdermal patches, and nicotine lozenges which are available over-the-counter, and nicotine nasal spray and nicotine inhalers, which are prescription products. All products have demonstrated efficacy as smoking cessation interventions. Nicotine gum and nicotine lozenges deliver nicotine faster than nicotine patches, while the patches can reduce early morning cravings if used overnight. The nicotine inhaler has the fewest side effect, and the nasal spray reduces cravings within a few minutes.
The nicotine mouth spray (NMS), delivers 1 mg nicotine per spray, thus enabling rapid nicotine absorption.
Nicotine chewing gum produces serum nicotine concentrations similar to those that occur during cigarette smoking and thereby to relieve the symptoms of nicotine withdrawal. Nicotine gum is available at doses of 4 mg and 2 mg with higher doses recommended for persons smoking over 15 cigarettes a day. The success rate achieved with nicotine gum at 6 months is 27% which is significantly higher than that achieved with placebo (18%).
The transdermal nicotine patch delivers a steady supply of nicotine to the bloodstream, thereby assisting in reducing the craving of nicotine. Individuals using a nicotine patch are twice as likely to quit as individuals using a placebo patch. Patches are available over the counter in 15- and 21-mg doses. Patches have a 6-month success rate of8-21% compared with 4-14% for placebo, and a 12-month success rate of 10-16% compared with 6-16% for placebo.
Nicotine lozenges are available in two forms. One form is a 2-mg nicotine bitartrate dihydrate sublingual tablet which delivers less nicotine than nicotine gum. It is effective in highly dependent but not in less dependent smokers. The other form is a 1-mg nicotine bitartrate salt lozenge. Compared with 2-mg or 4-mg nicotine gum, the nicotine lozenges deliver 25% to 27% more nicotine, because some nicotine is retained in the gum, whereas the lozenges dissolve completely and deliver their full dose. The 2-mg lozenge has a 6-week success rate of 46% compared with 30% for placebo and a 6-month success rate of 24% compared with 14% for placebo.

The nicotine mouth spray (NMS), delivers 1 mg nicotine per spray, thus enabling rapid nicotine absorption. A single-dose pharmacokinetic study reported a shorter time to maximum plasma nicotine concentration (10–12.5 min post-administration), and significantly higher area under the plasma-concentration time curve during the first 10 min, with 1 mg or 2 mg than either 4 mg nicotine lozenge or gum. The nicotine spray leads to significantly higher continuous abstinence rates than placebo from week 2 until week 6 (26.1% versus 16.1%).
The nicotine inhaler delivers nicotine in a form that is close to typical intake (inhaled by mouth), thus partially addressing the secondary reinforcement (sensory and ritual phenomena) important to a large subset of smokers. A single puff from the inhaler delivers ~13μg of nicotine at room temperature. To achieve 30% of the venous nicotine concentration achieved with 10 puffs of a cigarette in 5 minutes, it requires 80-100 puffs of the nicotine inhaler. Clinical studies have demonstrated odds ratios of 1.87-3.50 for successful at 6 months, and odds ratios of 1.59-3.04 at 1 year.
Novel nicotine and tobacco products: Can they be a complementary part of the solution?
Even with relatively low efficacy, therapeutic approaches to smoking cessation are worthwhile given their relative safety: If they help some people stop smoking, thereby incrementally reducing the costs of smoking-related morbidity and premature mortality, they are a sound policy “investment.” A number of policymakers have begun to consider if non-therapeutic options to encourage better lifestyle choices could complement the therapeutic approaches. For example, both the United Kingdom and New Zealand encourage smokers who are unable to quit smoking to switch to non-combusted “smoke-free” alternatives to cigarettes. Such products are not treated (or classified or reimbursed) as therapeutic goods but are regulated as tobacco or tobacco-related products, albeit ones that do not burn tobacco. In this section, we consider the health economics of such policies.
Electronic nicotine delivery systems or e-cigarettes
Electronic nicotine delivery systems (ENDS)(also called e-cigarettes or vaping products) consist of a battery that powers a heating element that aerosolizes a liquid that contains nicotine, flavorings and humectants.They produce a sensation that is similar to cigarette smoking. E-cigarettes are considered to be less harmful than conventional cigarette smoking, as they lack the harmful effects of tobacco combustion. Though not free from hazardous effects, the toxicity of e-cigarettes is lower than conventional cigarettes.
A report on e-cigarettes commissioned by Public Health England (PHE) notes that no other product delivers nicotine at a dose and speed that is similar to conventional cigarettes. Furthermore, absorption of the vapour released by e-cigarettes is slower than smoke of a conventional cigarette, and there is a low risk of nicotine overdose and inhalation of toxic doses of nicotine from an e-cigarette.
The WHO notes that tobacco use costs $500 billion each year in health care expenditures, productivity losses, fire damage, and other costs.
The composition of e-liquids requires strict regulation. Urine levels of hazardous compounds (PMA, HEMA, CNEMA, 3-HPMA, AAMA) are lower in users of e-cigarettes compared with users of e-cigarettes and conventional cigarettes. However, studies have also revealed that exposure to e-cigarettes containing nicotine leads to micro vascular endothelial dysfunction, increased oxidative stress and arterial stiffness. E-cigarette vapour also leads to cell death in adenocarcinomic human alveolar basal epithelial cells. Exposure to aerosols frome-liquids with or without nicotine is associated with neurotoxicity in animal studies.Notwithstanding these known hazards, there is a broad consensus that e-cigarettes, as a category, are significantly less hazardous that cigarettes.
Tobacco heating systems: A new technology with potential to reduce harm
Heated tobacco products or tobacco heating systems (THS) consist of electronic devices that heat processed tobacco rather than conventional burning, leading to the generation of aerosol that has fewer toxic chemicals than conventional cigarette smoke. The reduction in harmful and potentially harmful constituents (HPHC) is 92%. There is also a lower concentration of acetaldehyde, nicotine and other measured parameters over the background concentrations, in simulated settings of “residential”, hospitality” and “office”. The use of THS reduces the exposure to carboxyhemoglobin (COHb), S-PMA, MHBMA and 3-HPMA.
In a report by the United States Food and Drug Administration (FDA), it was reported that there is a reduction in systemic exposures to 15 biomarkers of exposure in persons switching from conventional cigarettes to THS. COHb was 4.65% to 6.66% at baseline, and reduced to 1.06-2.48% in the group switching from conventional cigarettes to THS group at 5 days. COHb levels were in the range of 4.5-6.07% among persons who continued to smoke, and 0.99-2.5% among those who abstained from smoking. Similar findings were noted for 3-HPMA, S-PMA and MHBMA. The report concluded that this reduced exposure may lead to a reduced likelihood of smoking-related diseases.
Clinical studies have demonstrated that switching from conventional cigarettes to THS led to significant improvement in high-density lipoprotein cholesterol, white blood cell count COHb, forced expiratory volume in 1 second (FEV1) and total NNAL. There is also a down regulation of genes involved in inflammatory responses and a reduced impact on proatherosclerotic markers. Based on extensive review of information, the FDA in its ruling on modified risk tobacco products (MRTP) declared that switching completely to THS significantly reduces your body’s exposure to HPHC. Furthermore, the report mentioned that “current evidence demonstrates that a measurable and substantial reduction in morbidity or mortality among individual tobacco users is reasonably likely in subsequent studies”.

Economic cost of smoking
Smoking has a substantial economic burden on society. According to the World Bank,~15% of the aggregate health care expenditure in high-income countries can be attributed to smoking. The WHO notes that tobacco use costs $500 billion each year in health care expenditures, productivity losses, fire damage, and other costs.
The economic burden of smoking on the national economy is usually expressed as a percentage of gross domestic product (GDP). The total economic costs of smoking-attributable diseases and deaths in 152 countries, representing 97% of the world’s smokers was estimated to be US$ 1436 billion in 2012, accounting for 1.8% of the world’s annual Gross Domestic Product (GDP) according to a study by WHO. In another meta-analysis, the economic burden of smoking was elucidated:
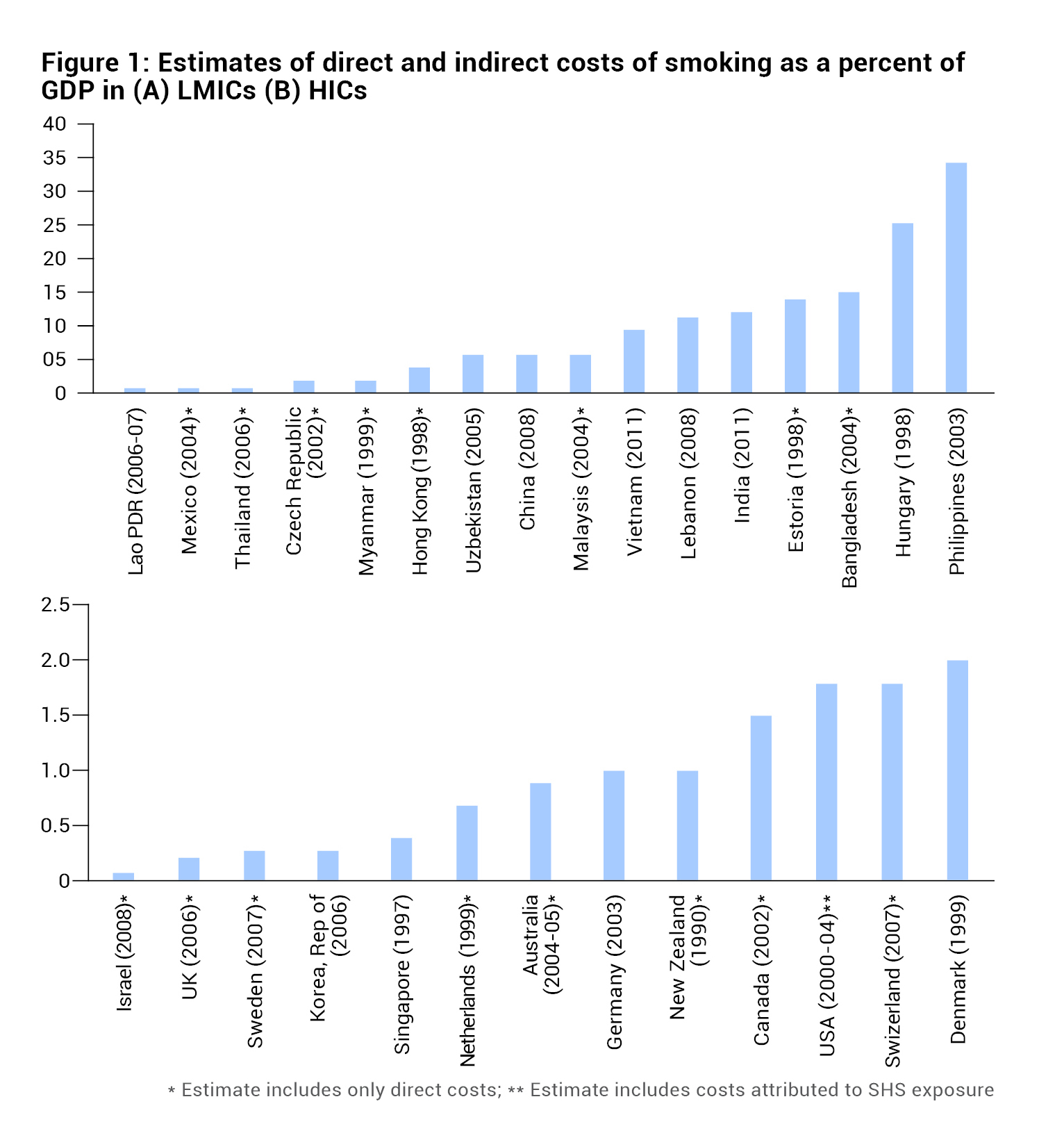
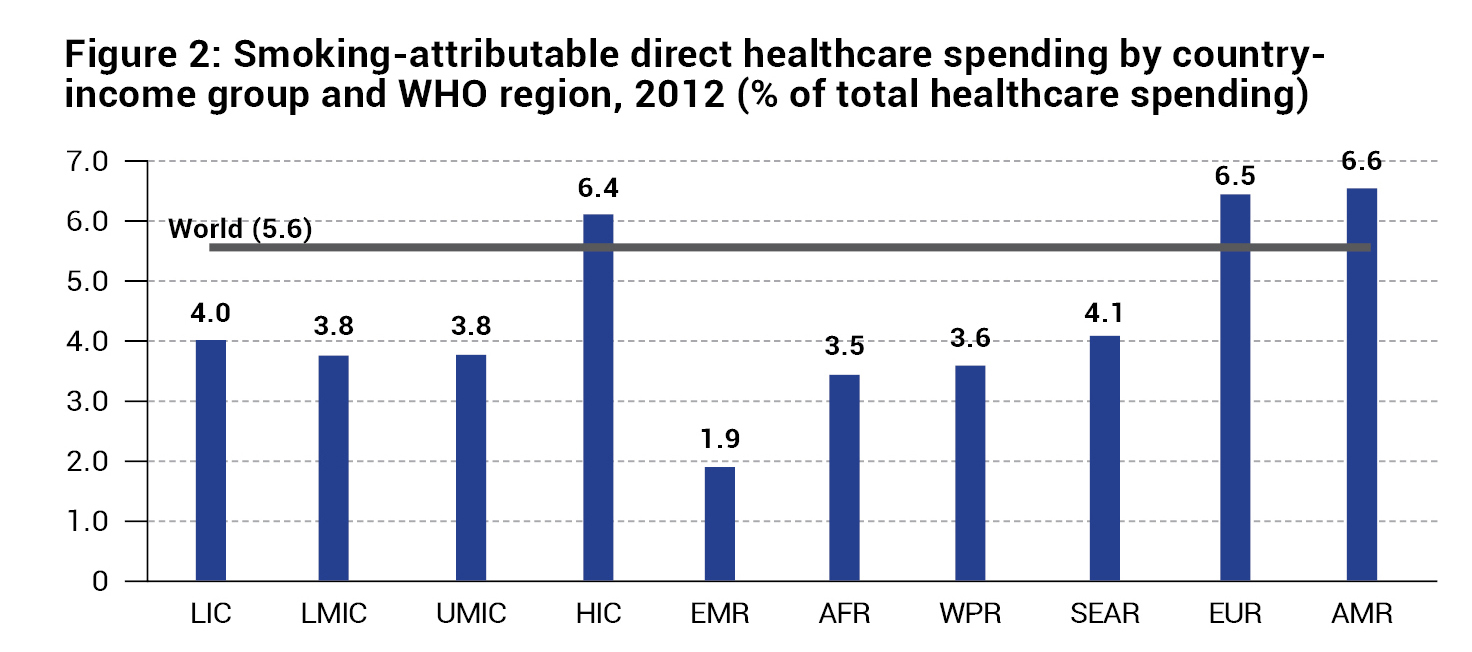
- The burden of smoking accounts for 0.22-0.82% of GDP.
- The direct cost attributable to smoking accounts for 1.5-6.8% of the national health care expenditure.
- The cost of smoking among adults is 6-14% of the personal expenditure on health care
- The cost of smoking-related premature mortality accounts for 52.6-90% of the total cost.
- The cost of passive smoking that accounts for 23% of the total cost of smoking.
- One study reported the total cost of smoking without including the cost of smoking-related premature mortality was 16% more than the total tax revenues collected from all tobacco products and many times higher than the total budget on tobacco control activities.
- Smoking attributable costs in low- and middle-income countries (LMICs) and high-income countries (HICs) is depicted in Figure 1, and smoking-attributable direct healthcare spending by country income group and WHO region is presented in Figure 2.
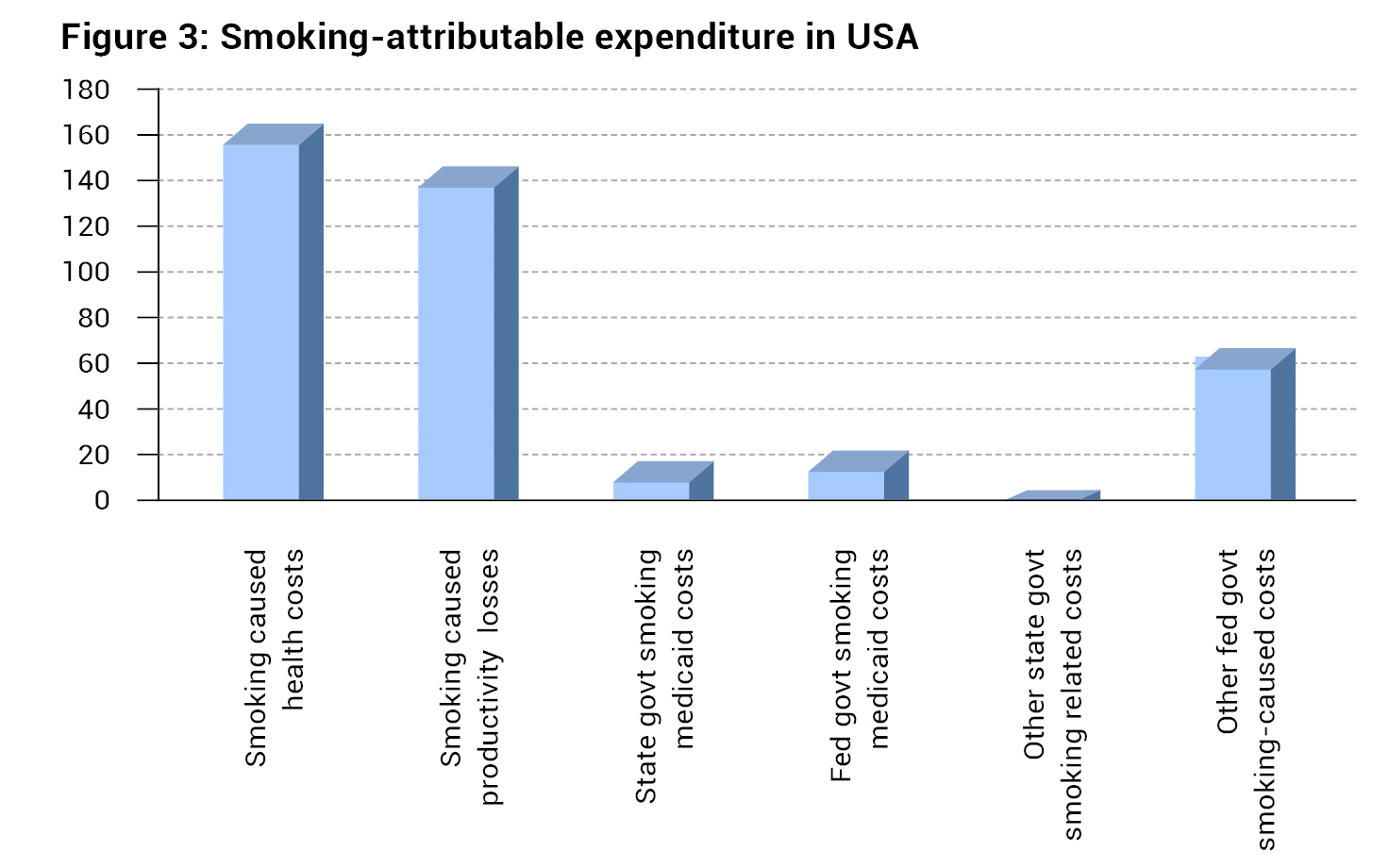
In USA, the total annual public and private health care expenditures caused by smoking is US$170 billion, amounting to ~1% of the GDP.
Data from India
A report on the economic burden of tobacco use in India in 2004 stated that the total cost of tobacco use was US$1.7 billion, which was nearly 4 times the expenditure on tobacco control. Tobacco-attributable direct costs were US$1.2 billion, which formed 4.7% of India’s healthcare expenditure. Smoked tobacco accounted for 77% of costs.
A recent study reported that the economic burden from tobacco accounted for 1% of India’s gross domestic product (GDP) in 2017-2018. The total economic costs attributable to tobacco use from all diseases for persons aged over 35 years was INR 1,773.4 billion (US$ 27.5 billion) or INR 3,772.5 per adult per year, of which 74% was attributed to smoking. Seventy-six percent of the cost of premature mortality is attributed to the use of tobacco. Direct medical costs accounted for 5.3% of total health expenditure. The direct economic costs attributable to second-hand smoke account for 8.1% of the total healthcare expenditure. Considering that nearly 40% of Indian adults are exposed to second-hand smoke, the findings of economic burden are a cause for concern.
Data from USA
In USA, the total annual public and private health care expenditures caused by smoking is US$170 billion, amounting to ~1% of the GDP. The proportion of health care expenditure attributable to smoking ranges between 6% and 18% across different states. As part of the indirect (non-health-related) costs of smoking, the total productivity losses caused by smoking each year in the US have been estimated at US$151 billion. The annual direct medical expenditure for early childhood respiratory illness attributable to maternal smoking is US$661 million for all children under the age of 6 years.
Data from UK
A number of studies have evaluated the cost of smoking in UK:
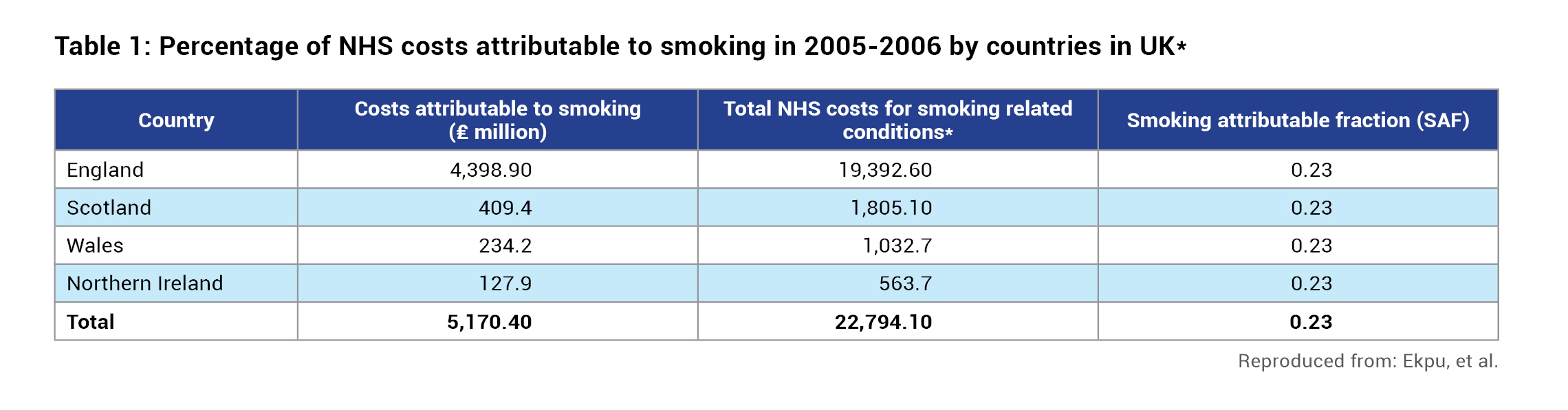
- Smoking-attributable costs to the NHS in 2006 was estimated at £2.7 billion. This includes smoking attributable hospital admissions (£1 billion), outpatient attendances (£190 million), general practitioner (GP) consultations (£530 million), practice nurse consultations (£50 million), and GP prescriptions (£900 million.
- The costs of smoking-induced ill health to the NHS was £5.2 billion in 2005–2006, representing about 5.5% of the total NHS budget that year.
Approximately 50 million working days are lost in UK annually due to smoking, valued at £1.71 billion. - The cost of treating childhood illnesses related to smoking is about £410 million per year.
Economic analysis of therapeutic interventions Lifestyle interventions
There are a number of non-pharmacological approaches to smoking cessation. Medical advice to quit produces 1-yr abstinence rates of up to 5–10%, which would have a significant public health impact if it were provided routinely. Self-help interventions, telephone-based counselling, group behaviour therapy and acupuncture are some of the approaches which have been evaluated.
Group-delivered acceptance and commitment therapy (ACT) and CBT had similar long-term quit rates. The 30-day PPA rates at the 12-month follow-up did not differ between group-delivered ACT and CBT (13.8% ACT vs. 18.1% CBT), thus making group-delivered ACT a viable alternative to CBT. One study has reported that contingency management (CM) along with cognitive behavioural therapy (CBT) has a higher rate of abstinence compared to CBT alone post-therapy (85.3% vs. 59.2%), even at 6 months follow-up (51.2% vs. 28.6%). CM is a form of behavioral treatment that typically provides financial incentives for abstinence.
In another study, a smartphone application was used for smoking cessation. A comparison of I Can Quit (an ACT-based smoking cessation application, which taught acceptance of smoking triggers), and the National Cancer Institute Quit Guide, a USCPG-based smoking cessation application (which taught avoidance of smoking triggers) showed that for the 30-day point prevalence abstinence (PPA) at the 12-month follow-up, I Can Quit participants had 1.49 times higher odds of quitting smoking compared with QuitGuide participants (28.2% vs. 21.1%; odds ratio [OR], 1.49; 95% CI, 1.22-1.83; p<0.001). Similar findings were noted for the 7-day PPA, at the 12-month follow-up, prolonged abstinence at the 12-month follow-up, abstinence from all tobacco products (including e-cigarettes) at the 12-month follow-up.
Nicotine replacement therapy
Nicotine patches
In a study by Fiscella, et al., the incremental cost-effectiveness of the addition of the nicotine patch to smoking cessation counselling was evaluated. The use of the patch produced 1 additional lifetime quitter at a cost of $7,332. The incremental cost-effectiveness of the nicotine patch by age group ranged from $4,390 to $10,943 per quality-adjusted life year (QALY) for men and $4,955 to $6,983 per QALY for women. A clinical strategy involving limiting prescription renewals to patients successfully abstaining for the first 2 weeks improved the cost-effectiveness of the patch by 25%.
In another study, Wasley, et al., reported that the average costs per year of life saved range from $965 to $1,585 for men and from $1,634 to $2,360 for women. Incremental costs per year of life saved range from $1,796 to $2,949 for men and from $3,040 to $4,391 for women.
Nicotine gum
Nicotine gum has proven efficacy as a cessation intervention in clinical trials. The use of nicotine gum is estimated to increase life expectancy by 5 years for men aged 35-39 years and by ~1.9 years for men aged 60-64 years. In women, the increase in life expectancy was 3.18 years for women aged 35-39 years, and 1.4 years for women aged 60-64 years. The cost per year of life saved with nicotine gum ranges from $4,113 to $6,465 for men and from $6,880 to $9,473 for women, depending on age. Cost-effectiveness was generally highest for patients who are between 45 and 54 years of age. At the time of this study, the findings were comparable to other commonly used medical practices, thus indicating that nicotine gum is a cost-effective strategy for smoking cessation.

E-cigarettes
The economic impact of policies that regulate and permit the same of e-cigarettes to smokers has been studied by a number of groups. In a study in UK evaluating the cost-effectiveness of e-cigarettes compared with NRT as a smoking aid, it was noted that the mean cost of treatment was £201 per participant in the NRT arm and £105 in the EC arm. In USA, 70% of the increased decline in cigarette smoking from 2013 to 2017 was associated with the rising use of e-cigarettes. The economic impact of the use of e-cigarettes has been studied, revealing a lowered annual per capita healthcare costs, compared to cigarette smokers and ex-smokers, for all age groups up to age 75.
- For people ages 25 to 44, the annual per capita healthcare costs of cigarette smokers are 9.8% greater than those of e-cigarette users, and the average annual per capita healthcare costs for ex-smokers are 19.8% greater than for e-cigarette users.
- For people ages 45 to 64, annual per capita healthcare spending for cigarette smokers is 8.8% greater than for e-cigarette users, and average per capita healthcare costs for ex-smokers are 34.4% greater than for e-cigarette users.
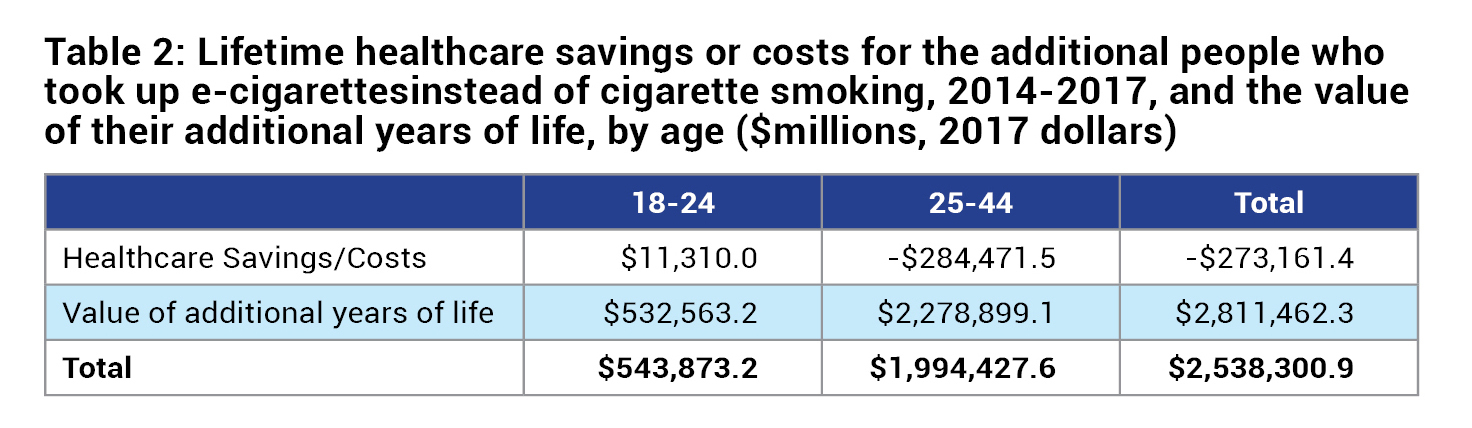
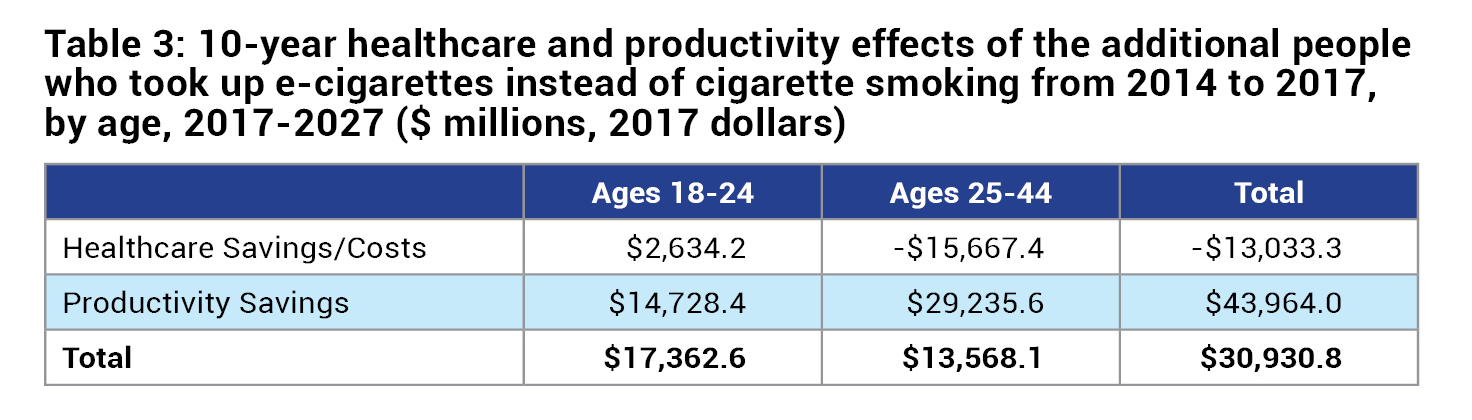
The use of e-cigarettes by the 922,301 people ages 18 to 24 in 2017, who otherwise would have started smoking cigarettes, should reduce their lifetime healthcare costs by $11.3 billion. E-cigarette users are on average $820 more productive per-year than ex-cigarette smokers and $2,371 more productive per-year than current smokers, and that ex-smokers who shifted to e-cigarettes are on average $1,554 more productive per-year than current smokers. Table 2 describes the healthcare saving and value of additional years of life for people who use e-cigarettes. Table 3 described the healthcare and productivity effects of e-cigarette use.
A study from USA has highlighted the potential of e-cigarettes in reducing deaths. This study used different models, including Status Quo scenario (cigarette use), Optimistic Scenario (published harm reduction of e-cigarettes was considered), Pessimistic Scenario (‘worst case ‘of suggested harms; e-cigarettes are more harmful than science indicates), and compared it to a Substitution model (switching from cigarettes to e-cigarettes). The study reported that compared with the Status Quo, replacement of cigarette by e-cigarette use over a 10-year period yields 6.6 million fewer premature deaths with 86.7 million fewer life years lost in the Optimistic Scenario. Under the Pessimistic Scenario, 1.6 million premature deaths are averted with 20.8 million fewer life years lost. The study concluded that even with conservative estimates, switching from cigarettes to e-cigarettes can lead to substantial gains in life-years. It has been estimated that the use of vaporized nicotine products could lead to a 21% reduction in smoking-attributable deaths, and a 20% reduction in life years lost. It is important to note that policies regulating the use of e-cigarettes should discourage the use of these products in never smokers, and rather use this product as a smoking cessation intervention.
Pharmacological interventions
Varenicline
Varenicline is a highly effective smoking cessation aid that competes with nicotine to activate receptors, thus reducing withdrawal symptoms and rewarding effects of smoking. In male smokers in Japan, varenicline reduces medical costs by ¥43,846 and increased QALYs by 0.094 compared with placebo. In female smokers, varenicline increased lifetime medical costs by ¥12,115 and QALY by 0.03. The ICER was ¥346,143 per QALY gained. When analyzed for all patients varenicline reduced lifetime medical costs by ¥29,220 and increased QALY by 0.079. When applied to the whole population that would use varenicline, the initial incremental cost for treatment was ¥14.2 billion, and future saving for treatment cost of tobacco-associated diseases was ¥23.7 billion, leading to overall saving was ¥9.5 billion.
Varenicline is more cost-effective than either NRT or bupropion in the UK and elsewhere. The cost per additional quitter for varenicline is approximately £2,170.31 The ICER of varenicline compared with no pharmacotherapy has been estimated at between £950 and £1,140 per QALY gained. Furthermore, a longer treatment duration of 24 weeks, compared with 12 weeks, is also cost-effective, resulting in an ICER of £622 per QALY.
Bupropion
Bupropion (an atypical antidepressant) acts on dopamine and noradrenaline pathways and is likely to be a nicotinic antagonist. It reduces symptoms of withdrawal as well as the rewarding effects of smoking, leading to 70% higher smoking cessation rates compared with placebo. Economic analysis revealed that cost per life-time quitter for bupropion users is £964 and £1,799 (as of 2002). The ICER of bupropion is £830 per QALY in USA in 2005 (compared with brief advice), and £316 to £2,212 in the UK.
In a comparison of bupropion with transdermal patches from an employers’ perspective, bupropion is more cost-beneficial than either NTP or bupropion/NTP, with a net benefit in the first post-quit year of up to $338 per employee, as compared to $26 for NTP and $178 for NTP plus bupropion.

Comparison of NRT and pharmacological interventions: Findings of the Norwegian Knowledge Centre for the Health Services
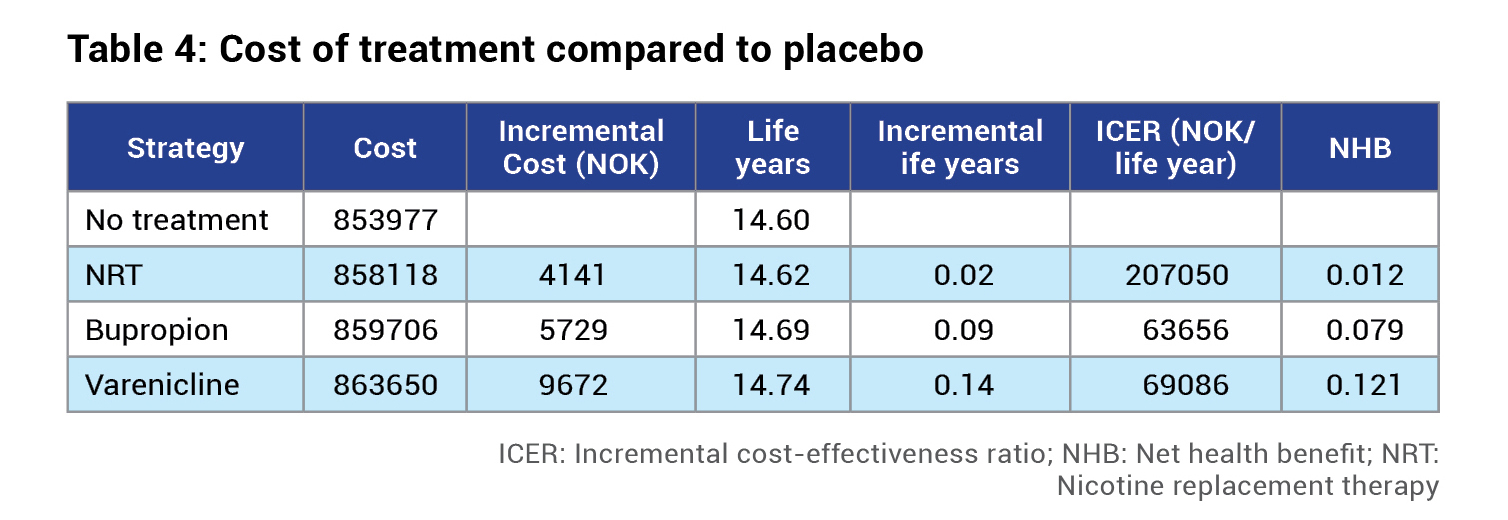
The evaluation of the cost-effectiveness of drugs for smoking cessation in a Norwegian setting has demonstrated that NRT, bupropion and varenicline are all cost-effective. NRT, bupropion and varenicline yield 0.02, 0.09 and 0.14 additional life-years, at an additional cost of NOK 4,141, NOK 5,729 and NOK 9,672, respectively (Table 4). Compared to no treatment, bupropion gives 0.09 additional life-years at an additional cost of NOK 5,729. Compared to bupropion, varenicline gives 0.05 additional life-years at an additional cost of NOK 3,944.
Conclusion
In the current scenario of nicotine addiction, wherein there is a significant health and economic burden across the globe, it is becoming increasingly necessary to focus on smoking cessation interventions that provide individuals the support to quit smoking. The interventions have to be effective in reducing the craving to smoke, while also being cost-effective. The nonpharmacological approaches such as CBT have high rates of abstinence after intervention, which tend to drop at 12 months after the intervention. Novel methods such as the use of smartphone apps have also shown efficacy, with higher odds of abstinence reported from clinical trials. A number of nicotine replacement therapies, pharmacological therapies as well as electronic cigarettes have demonstrated cost-effectiveness. E-cigarettes have shown potential in reducing the number of deaths and life-years lost, while also leading to saving of healthcare costs.
There is an also potential health benefit, such as reduced exposure to nicotine, lower risk of overdose, and lower exposure to hazardous compounds reported for e-cigarettes. Similarly, tobacco heating systems also lead to reduced exposures to hazardous compounds, and a reduced inflammatory response. This lends support to the use of these products as smoking cessation interventions. Similarly, the development of tobacco heating systems, which are known to have lower levels of nicotine and toxic chemicals, could also be expected to have a beneficial economic impact.
(The author is MD (Pharmacology), Certified in Patient Safety (Johns Hopkins University, USA), FISQua (U.K), NABH Assessor for Hospitals and Ethics Committee, Formerly attached to Stanford University School of Medicine, USA)

